Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -ray irradiation in HNO
-ray irradiation in HNO media
mediaNogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 296(1), p.423 - 427, 2013/04
Times Cited Count:5 Percentile:38.34(Chemistry, Analytical)Stability of polyvinylpolypyrrolidone (PVPP), a resin with adsorption selectivity to U(VI) in nitric acid media, against  -ray irradiation has been examined using HNO
-ray irradiation has been examined using HNO solutions of various concentrations. As the result, no clear decrease in the capacity was observed for any samples. Or rather, it was found that the capacity increased by approximately 50% for the PVPP slurry irradiated in 6 M HNO
solutions of various concentrations. As the result, no clear decrease in the capacity was observed for any samples. Or rather, it was found that the capacity increased by approximately 50% for the PVPP slurry irradiated in 6 M HNO . The infrared spectroscopic study indicates that PVPP degrades by
. The infrared spectroscopic study indicates that PVPP degrades by  -ray irradiation in HNO
-ray irradiation in HNO from the cleavage of the pyrrolidone ring by the addition of oxygen atom originating from HNO
from the cleavage of the pyrrolidone ring by the addition of oxygen atom originating from HNO , followed by the formation of chain monoamides with multiple coordinative atoms by the continuous addition of oxygen, finally leading to the generation of primary-amine type anion exchange resin. It is also indicated that all generated functional groups possess adsorptivity to U(VI) in 3 M HNO
, followed by the formation of chain monoamides with multiple coordinative atoms by the continuous addition of oxygen, finally leading to the generation of primary-amine type anion exchange resin. It is also indicated that all generated functional groups possess adsorptivity to U(VI) in 3 M HNO .
.
 -ray irradiation
-ray irradiationNogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Science China; Chemistry, 55(9), p.1739 - 1745, 2012/09
Times Cited Count:4 Percentile:19.62(Chemistry, Multidisciplinary)Stability of N-alkylated pyrrolidone derivatives (NRPs) against radiation was examined by irradiation tests with  Co
Co  -ray. We have been developed a novel reprocessing system using NRPs which have precipitation ability to haxa- and tetravalent actinides in nitric acid media. Degradation rates of NRPs are evaluated by irradiation in 3M nitric acid solutions and mechanism of degradation are discussed in the present paper.
-ray. We have been developed a novel reprocessing system using NRPs which have precipitation ability to haxa- and tetravalent actinides in nitric acid media. Degradation rates of NRPs are evaluated by irradiation in 3M nitric acid solutions and mechanism of degradation are discussed in the present paper.
Iwata, Mitsunao*; Sugiyama, Yuichi*; Murata, Yoshinori*; Takaya, Shigeru
Defect and Diffusion Forum, 326-328, p.578 - 582, 2012/04
Times Cited Count:0 Percentile:0.01(Nanoscience & Nanotechnology)no abstracts in English
Nogami, Masanobu*; Harada, Masayuki*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Progress in Nuclear Energy, 53(7), p.948 - 951, 2011/09
Times Cited Count:3 Percentile:25.9(Nuclear Science & Technology)The precipitation ability of 1,3-dimethyl-2-imidazolidone (DMI) to U(VI) and U(IV) was examined using nitric acid solutions. While DMI precipitated U(VI) from 3 M nitric acid, no precipitate was observed in the solution containing 0.15 M U(IV) and 3 M nitric acid by adding DMI at the ratio of [DMI]/[U(IV)]=5. This indicates that the selectivity of DMI to U(VI) than U(IV). In spite of the excellent selectivity to U(VI), DMI has a disadvantage on the stability in nitric acid, because gradual acid hydrolysis of DMI is inevitable due to the nature of the chemical structure. Experiments on the stability of DMI in  -ray irradiation and heating in nitric acid solutions showed that the stability is strongly affected by the concentration of nitric acid and that DMI may be applicable in nitric acid solutions up to ca. 2 M.
-ray irradiation and heating in nitric acid solutions showed that the stability is strongly affected by the concentration of nitric acid and that DMI may be applicable in nitric acid solutions up to ca. 2 M.
Nogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 283(2), p.541 - 546, 2010/02
Times Cited Count:20 Percentile:78.67(Chemistry, Analytical)Adsorptivity of polyvinylpolypyrrolidone (PVPP) to various metal ions was examined as a part of the development of resins with selectivity to U(VI) in nitric acid media. It was found that PVPP has a strong adsorptivity to U(VI) in wide concentration range of nitric acid. The mechanism of U(VI) adsorption by PVPP was discussed by results of Scatchard plot analysis and infrared spectroscopic analysis. Furthermore, it was found that fission productions except for Re(VII) as the simulant of Tc(VII) and Pd(II) are not adsorbed on to PVPP and that Pd(II) and Re(VII) species are weakly adsorbed in the lower concentration ranges of nitric acid, where the adsorption rates of Pd(II) are extremely slower than those of U(VI). These results indicate that U(VI) can be separated from other metal ions by PVPP.
 -ray irradiation
-ray irradiationNogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Kawata, Yoshihisa; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
JAEA-Review 2009-041, JAEA Takasaki Annual Report 2008, P. 25, 2009/12
As a part of the development of a novel reprocessing system for spent FBR fuels based on the precipitation method, influence of concentrations of HNO on the stability by
on the stability by  -ray irradiation was examined for
-ray irradiation was examined for  -
- -butyl-2-pyrrolidone (NBP), a candidate precipitant for the first precipitation step for selectively precipitating U(VI). The residual ratios of the samples for HNO
-butyl-2-pyrrolidone (NBP), a candidate precipitant for the first precipitation step for selectively precipitating U(VI). The residual ratios of the samples for HNO solutions up to 3 M were found to be decreased identically, where ca. 20% of NBP was degraded after the irradiation of 1 MGy. It was found that the degradation of the samples of 6 M HNO
solutions up to 3 M were found to be decreased identically, where ca. 20% of NBP was degraded after the irradiation of 1 MGy. It was found that the degradation of the samples of 6 M HNO is more distinguished, where ca. 30% was degraded after the irradiation of 0.1 MGy. As the result of the investigation of the degradation mechanism of NBP, it was revealed that the degradation started from the cleavage of the pyrrolidone ring of NBP by the addition of oxygen atom, followed by the formation of chain monoamides and C4 compounds by the continuous addition of oxygen, leading to the generation of oxalic acid.
is more distinguished, where ca. 30% was degraded after the irradiation of 0.1 MGy. As the result of the investigation of the degradation mechanism of NBP, it was revealed that the degradation started from the cleavage of the pyrrolidone ring of NBP by the addition of oxygen atom, followed by the formation of chain monoamides and C4 compounds by the continuous addition of oxygen, leading to the generation of oxalic acid.
 -Alkyl-2-pyrrolidone derivatives (Alkyl =
-Alkyl-2-pyrrolidone derivatives (Alkyl =  -propyl,
-propyl,  -butyl,
-butyl, 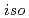 -butyl, and cyclohexyl)
-butyl, and cyclohexyl)Takao, Koichiro*; Noda, Kyoko*; Nogami, Masanobu*; Sugiyama, Yuichi*; Harada, Masayuki*; Morita, Yasuji; Nishimura, Kenji*; Ikeda, Yasuhisa*
Journal of Nuclear Science and Technology, 46(10), p.995 - 999, 2009/10
Times Cited Count:14 Percentile:66.87(Nuclear Science & Technology)We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. In the present study, the solubility of UO (NO
(NO )
) (NRP)
(NRP) (NRP =
(NRP =  -alkyl-2-pyrrolidone, alkyl =
-alkyl-2-pyrrolidone, alkyl =  -propyl,
-propyl,  -butyl,
-butyl, 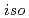 -butyl and cyclohexyl) in aqueous solutions with HNO
-butyl and cyclohexyl) in aqueous solutions with HNO has been examined. As a result, the solubility of each species of UO
has been examined. As a result, the solubility of each species of UO (NO
(NO )
) (NRP)
(NRP) generally decreases with increasing concentrations of HNO
generally decreases with increasing concentrations of HNO and NRP (
and NRP (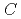 (HNO
(HNO ) and
) and 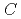 (NRP), respectively). The solubility of UO
(NRP), respectively). The solubility of UO (NO
(NO )
) (NRP)
(NRP) also depends on the type of NRP; a higher hydrophobicity of NRP generally leads to a lower solubility of UO
also depends on the type of NRP; a higher hydrophobicity of NRP generally leads to a lower solubility of UO (NO
(NO )
) (NRP)
(NRP) . The logarithms of effective solubility products (
. The logarithms of effective solubility products (
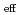 ) of UO
) of UO (NO
(NO )
) (NRP)
(NRP) at different
at different 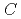 (HNO
(HNO ) values and 293 K were evaluated.
) values and 293 K were evaluated.
Washiya, Tadahiro; Sano, Yuichi; Komaki, Jun; Funasaka, Hideyuki; Sugiyama, Toshihide
no journal, ,
no abstracts in English
Noda, Kyoko*; Takao, Koichiro*; Sugiyama, Yuichi*; Harada, Masayuki*; Nogami, Masanobu*; Maruyama, Koichi*; Takahashi, Hiroaki*; Kim, S.-Y.; Sato, Makoto; Mineo, Hideaki; et al.
no journal, ,
We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. In previous investigation, N-cyclohexyl-2-pyrrolidone (NCP) is used as a precipitant, which is able to precipitate selectively UO
 ions in HNO
ions in HNO solution, and a process consisting of two separation steps; selective U precipitation step and U-Pu co-precipitation step, was developed. In order to make the process more effective and more economical, we are now studying precipitation of U and Pu with other pyrrolidone derivatives. The outline of the study and main results obtained until now are shown in this presentation.
solution, and a process consisting of two separation steps; selective U precipitation step and U-Pu co-precipitation step, was developed. In order to make the process more effective and more economical, we are now studying precipitation of U and Pu with other pyrrolidone derivatives. The outline of the study and main results obtained until now are shown in this presentation.

 -precipitant from a viewpoint of crystallography
-precipitant from a viewpoint of crystallographyTakao, Koichiro*; Noda, Kyoko*; Nogami, Masanobu*; Sugiyama, Yuichi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Morita, Yasuji; Nishimura, Kenji*
no journal, ,
We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. Structural analyses of UO (NO
(NO )
) L
L (L=11 kinds of pyrrolidone derivatives) have been carried out using X-ray diffraction method to obtain some useful data for the selection of the optimum precipitant. It was found that the U(VI) complex with N-iso-butyl-2-pyrrolidone makes the most compact crystal.
(L=11 kinds of pyrrolidone derivatives) have been carried out using X-ray diffraction method to obtain some useful data for the selection of the optimum precipitant. It was found that the U(VI) complex with N-iso-butyl-2-pyrrolidone makes the most compact crystal.
Takao, Koichiro*; Noda, Kyoko*; Nogami, Masanobu*; Sugiyama, Yuichi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Morita, Yasuji; Nishimura, Kenji*
no journal, ,
Precipitation behavior of U(VI) and some fission products (FP) with pyrrolidone derivatives of N-(1,2-dimethyl)propyl-2-pyrrolidone (NDMProP) and N-neopenthyl-2-pyrrolidone (NNpP) in the solutions of U-FP mixture has been examined in order to evaluate their applicability to the U-Pu co-precipitation process in the reprocessing based only on precipitation method. We have previously developed a process with N-cyclohexyl-2-pyrrolidone (NCP). It was found that U(VI) was precipitated in a high yield with any of the three precipitants and the order of the precipitation ability for U(VI) was NCP NNpP
NNpP NDMProP. Decontamination factors (DF) against FP except Zr(IV) and Mo(VI) in the U(VI) precipitation were over 100, and therefore NNpP and NDMProP can be used as an alternative precipitants to NCP.
NDMProP. Decontamination factors (DF) against FP except Zr(IV) and Mo(VI) in the U(VI) precipitation were over 100, and therefore NNpP and NDMProP can be used as an alternative precipitants to NCP.
Nogami, Masanobu*; Noda, Kyoko*; Takao, Koichiro*; Sugiyama, Yuichi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Kawata, Yoshihisa; Morita, Yasuji; Nishimura, Kenji*
no journal, ,
We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. The present study deals with durability of new precipitants with low and high hydrophobicity against  -irradiation and heating. Results showed that the precipitants with low hydrophobicity, N-n-butyl-2-pyrrolidone, N-propyl-2-pyrrolidone and N-iso-butyl-2-pyrrolidone, have enough durability against
-irradiation and heating. Results showed that the precipitants with low hydrophobicity, N-n-butyl-2-pyrrolidone, N-propyl-2-pyrrolidone and N-iso-butyl-2-pyrrolidone, have enough durability against  -irradiation. The precipitants with high hydrophobicity, N-cyclohexyl-2-pyrrolidone, N-(1,2-dimethyl)propyl-2-pyrrolidone and N-neopenthyl-2-pyrrolidone, also have enough durability but gave lower precipitation yield when they irradiated with higher dose rate. The precipitation ability of all the precipitants did not changed by the heating at 50
-irradiation. The precipitants with high hydrophobicity, N-cyclohexyl-2-pyrrolidone, N-(1,2-dimethyl)propyl-2-pyrrolidone and N-neopenthyl-2-pyrrolidone, also have enough durability but gave lower precipitation yield when they irradiated with higher dose rate. The precipitation ability of all the precipitants did not changed by the heating at 50 C for three days.
C for three days.
Nogami, Masanobu*; Takao, Koichiro*; Sugiyama, Yuichi*; Noda, Kyoko*; Harada, Masayuki*; Ikeda, Yasuhisa*; Morita, Yasuji; Nishimura, Kenji*
no journal, ,
We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. The present study deals with the effect of addition of masking agents on co-precipitation of Pu(IV) in the selective U(VI) precipitation. It was found that precipitation of U(IV), which was used as substitute for Pu(IV), was suppressed by the presence of acetohydoxamic acid.
Nogami, Masanobu*; Kawasaki, Takeshi*; Takao, Koichiro*; Noda, Kyoko*; Sugiyama, Yuichi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Morita, Yasuji; Chikazawa, Takahiro*; Kikuchi, Toshiaki*; et al.
no journal, ,
A reprocessing system for spent FBR fuels based on the two precipitation processes has been proposed. In this system, first only U(VI) species are precipitated using pyrrolidone derivatives (NRP) with low hydrophobicity and donicity, and secondly residual U(VI) and Pu(IV, VI) are precipitated simultaneously using pyrrolidone derivative with high precipitation ability. In order to develop such a reprocessing method, behavior of uranyl ions in HNO has been studied using various novel NRP. In this presentation, recent advances on precipitation behavior and of uranyl ions and calcination of U(VI)-NRP will be introduced.
has been studied using various novel NRP. In this presentation, recent advances on precipitation behavior and of uranyl ions and calcination of U(VI)-NRP will be introduced.
Kawasaki, Takeshi*; Nogami, Masanobu*; Sugiyama, Yuichi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Morita, Yasuji; Kikuchi, Toshiaki*
no journal, ,
Recovery of the precipitant by evaporation of the precipitates of U(VI) with pyrrolidone derivatives was investigated for the development of an advanced reprocessing system for spent FBR fuels based only on precipitation method. It was found that the U(VI) precipitate with N-n-butyl-2-pyrrolidone (NBP) melted at about 100 C and the bubble formation was observed at 140 - 150
C and the bubble formation was observed at 140 - 150 C. Most of the recovered materials by cooling the evaporated fraction was NBP, and therefore, it is expected to recover the precipitant without degradation by this methods.
C. Most of the recovered materials by cooling the evaporated fraction was NBP, and therefore, it is expected to recover the precipitant without degradation by this methods.
Sugiyama, Yuichi*; Nogami, Masanobu*; Kawasaki, Takeshi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Morita, Yasuji; Kikuchi, Toshiaki*
no journal, ,
We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. The present study deals with recovery of residual U(VI) from high-level liquid waste by adsorption with polyvinylpolypyrrolidone (PVPP) after the main precipitation step. Batch experiments showed the high distribution coefficient (Kd) for U(VI) in the wide range of nitric acid concentration and higher Kd at lower nitric acid concentration. Among fission product elements, only Pd(II) was adsorbed weekly, but in the column experiment Pd was separated from U(VI) by washing the adsorbent with 3M nitric acid.
Nogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Kawata, Yoshihisa; Morita, Yasuji; Kikuchi, Toshiaki*
no journal, ,
We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. In the present study, the durability of N-n-butyl-2-pyrrolidone (NBP) against  -irradiation and heat and the mechanism of degradation were examined in the various conditions. NBP is the most promising candidate as the precipitant for the first step in which U is selectively separated. The results showed that many kinds of multi-functional degradation products were formed by irradiation and heating and therefore that the influence of these degradation products on the following step in the reprocessing system should be investigated in detail.
-irradiation and heat and the mechanism of degradation were examined in the various conditions. NBP is the most promising candidate as the precipitant for the first step in which U is selectively separated. The results showed that many kinds of multi-functional degradation products were formed by irradiation and heating and therefore that the influence of these degradation products on the following step in the reprocessing system should be investigated in detail.
Harada, Masayuki*; Nogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Ikeda, Yasuhisa*; Morita, Yasuji; Kikuchi, Toshiaki*
no journal, ,
We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. In the present study, the effects of addition of masking agent for Pu(IV) in the first selective U precipitation step were investigated to suppress the Pu co-precipitation using U(IV) as a substitute for Pu(IV). The results showed that amide compounds such as N, N-dimethyl-acetamide have relatively high masking ability.
Nogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Kikuchi, Toshiaki*; Kawata, Yoshihisa; Morita, Yasuji
no journal, ,
In the novel reprocessing system under development for spent FBR fuels based on the precipitation method, U and Pu of very low concentrations remain in the supernatant after almost all U and Pu in the dissolvent solution are separated by precipitation. The remaining U and Pu are supposed to be removed by an adsorption method using polyvinylpolypyrrolidone (PVPP) which is insoluble in water. In this study, stability of PVPP in HNO solutions under
solutions under  -ray irradiation was investigated. As the result, remarkable decreases in the capacity were not found in all samples. Or rather, the capacity was found to be increased for the sample irradiated in 6M HNO
-ray irradiation was investigated. As the result, remarkable decreases in the capacity were not found in all samples. Or rather, the capacity was found to be increased for the sample irradiated in 6M HNO for 0.90 MGy. These facts revealed that PVPP maintains the adsorptivity to U(VI) under various concentrations of HNO
for 0.90 MGy. These facts revealed that PVPP maintains the adsorptivity to U(VI) under various concentrations of HNO for 10 days of operation under
for 10 days of operation under  -ray irradiation.
-ray irradiation.
Sugiyama, Yuichi*; Iwata, Mitsunao*; Murata, Yoshinori*; Takaya, Shigeru
no journal, ,
no abstracts in English