Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Matsui, Hiroshi; Ikezawa, Yoshio; Yoshida, Yoshikazu*; *; *; *; *
Aerosols: Science,Industry,Health and Environment,Vol. 2, p.720 - 723, 1990/00
no abstracts in English
Eguchi, Ayano; Okamura, Hiroyuki; Sugita, Tsuyoshi; Ueda, Yuki; Morita, Kotaro*; Shimojo, Kojiro; Naganawa, Hirochika; Hirayama, Naoki*
no journal, ,
In this study, the effect of the hydrophobicity and structure of an ionic liquid (IL) anion on the extraction of trivalent lanthanoid ions (Ln(III) = La, Nd, Eu, Dy, Lu) using 2-thenoyltrifluoroacetone (Htta) was investigated. Four 1-butyl-3-methylimidazolium bis(perfluoroalkanesulfonyl)imides with different perfluoroalkyl (Rf) chain length (n=0, 1, 2, 4) were synthesized and used as the extraction solvent. The extraction selectivity of Ln(III) was in the order of Lu 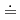 Dy
Dy  Eu
Eu  Nd
Nd  La for all ILs. In addition, there was no significant difference in the extractability among ILs. The extracted species of each Ln(III) in these IL systems were investigated based on the slope of the plots of the logarithmic distribution ratio of Ln(III) as a function of aqueous phase pH. The results implied that the extracted species can be adjusted by Rf chain length of IL anions.
La for all ILs. In addition, there was no significant difference in the extractability among ILs. The extracted species of each Ln(III) in these IL systems were investigated based on the slope of the plots of the logarithmic distribution ratio of Ln(III) as a function of aqueous phase pH. The results implied that the extracted species can be adjusted by Rf chain length of IL anions.
Eguchi, Ayano; Okamura, Hiroyuki; Sugita, Tsuyoshi; Ueda, Yuki; Morita, Kotaro*; Shimojo, Kojiro; Naganawa, Hirochika; Hirayama, Naoki*
no journal, ,
In this study, the effect of the perfluoroalkyl (Rf) chain length of the ionic liquid (IL) anion on the extraction of trivalent lanthanoid ions (Ln(III) = La, Nd, Eu, Dy, Lu) using 2-thenoyltrifluoroacetone was investigated. Four 1-butyl-3-methylimidazolium bis(perfluoroalkanesulfonyl)imides with different Rf chain length (n = 1-4) were synthesized and used as the extraction solvent. There was no significant difference in the extractability among ILs. The extracted species of each Ln(III) in these IL systems were investigated based on the slope of the plots of the logarithmic distribution ratio of Ln(III) as a function of aqueous phase pH. It was revealed that the extracted complex depends on even or odd number of the Rf chain length n.
Eguchi, Ayano; Okamura, Hiroyuki; Sugita, Tsuyoshi; Ueda, Yuki; Morita, Kotaro*; Shimojo, Kojiro; Naganawa, Hirochika; Hirayama, Naoki*
no journal, ,
In this study, the effect of the perfluoroalkyl (Rf) chain length of the ionic liquid (IL) anion on the IL chelate extraction was investigated. Four 1-butyl-3-methylimidazolium bis(perfluoroalkanesulfonyl)imides with different Rf chain length (n = 1 - 4) were synthesized and used as extraction solvents. Herein, the extraction of trivalent lanthanoids (Ln(III) = La, Nd, Eu, Dy and Lu) with an extractant, 2-thenoyltrifluoroacetone, was studied. It was revealed that the extracted complex depends on even or odd number of the Rf chain length n.
Eguchi, Ayano; Okamura, Hiroyuki; Sugita, Tsuyoshi; Ueda, Yuki; Morita, Kotaro*; Shimojo, Kojiro; Naganawa, Hirochika; Hirayama, Naoki*
no journal, ,
In this study, the effect of the perfluoroalkyl (Rf) chain length of the ionic liquid (IL) anion on the IL chelate extraction of trivalent lanthanoids using 2-thenoyltrifluoroacetone was investigated. Four 1-butyl-3-methylimidazolium bis(perfluoroalkanesulfonyl)imides with different Rf chain length (n = 1-4) were synthesized and used as extraction solvents. The extracted species differed depending on odd-even number of the Rf chain length n, and it was found that the Rf chain length of IL anion has the odd-even effect on the extracted species. The fluorescence lifetime of the extracted Eu(III) complex was measured and the number of coordinating water of Eu(III) was determined. As a result, the Eu(III) complex did not have coordinating water. It was suggested that the Rf chain length of the IL anion does not affect the number of coordinating water of the extracted complex.
Eguchi, Ayano; Okamura, Hiroyuki; Sugita, Tsuyoshi; Ueda, Yuki; Morita, Kotaro*; Shimojo, Kojiro; Naganawa, Hirochika; Hirayama, Naoki*
no journal, ,
Physical and chemical properties of ionic liquids (ILs) can be tuned by changing the combination of an anion and a cation. In the solvent extraction of metal ions, it is well known that the structure and alkyl chain length of the IL cation have a great effect on the extractability. However, the structural effect of the IL anion on the extractability is hardly clarified. In this study, the effect of the side chain length of the IL anion on the IL chelate extraction of trivalent lanthanoids using 2-thenoyltrifluoroacetone was investigated. Four 1-butyl-3-methylimidazolium bis(perfluoroalkanesulfonyl)imides with different side chain lengths (n = 1-4) were synthesized and used as extraction solvents. When using ILs which side chain lengths n were even (n = 2, 4), the extracted species were different from those when using ILs that n were odd (n = 1, 3) and the IL having hexafluorophosphate anion. It was, therefore, implied that the extracted species can be controlled by the side chain length of the IL anion.
Eguchi, Ayano; Okamura, Hiroyuki; Sugita, Tsuyoshi; Ueda, Yuki; Morita, Kotaro*; Shimojo, Kojiro; Naganawa, Hirochika; Hirayama, Naoki*
no journal, ,
Physical and chemical properties of ionic liquids (ILs) can be tuned by changing the combination of an anion and a cation composing an IL. In the solvent extraction of metal ions, it is considered that IL anions affect extraction by the interaction IL anions and metal ions. However, the structural effect of the IL anions on extraction is hardly clarified. In this study, the effect of the side chain of the IL anions on the IL chelate extraction of trivalent lanthanoids using 2-thenoyltrifluoroacetone was investigated. Four 1-butyl-3-methylimidazolium bis(perfluoroalkanesulfonyl)imides with different carbon number of side chain n (n = 1-4) were synthesized and used as extraction solvents. When n was even, the extracted species were different from those when n was odd. This result was considered to be due to the different IL solvation effects on the extracted complexes depending on n. It was implied that the side chain in the IL anions was involved in the extraction mechanism by affecting solvation of extracted complexes.