Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Cm +
Cm +  O reaction
O reactionMurakami, Masashi*; Goto, Shinichi*; Murayama, Hirofumi*; Kojima, Takayuki*; Kudo, Hisaaki*; Kaji, Daiya*; Morimoto, Koji*; Haba, Hiromitsu*; Kudo, Yuki*; Sumita, Takayuki*; et al.
Physical Review C, 88(2), p.024618_1 - 024618_8, 2013/08
Times Cited Count:15 Percentile:66.57(Physics, Nuclear)Production cross sections of Rf isotopes in the  Cm +
Cm +  O reaction were measured at the beam energy range of 88.2 to 101.3 MeV by use of a gas-filled recoil ion separator. The excitation functions of
O reaction were measured at the beam energy range of 88.2 to 101.3 MeV by use of a gas-filled recoil ion separator. The excitation functions of 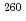 Rf,
Rf, 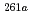 Rf, and
Rf, and  Rf were obtained together with those of spontaneously fissioning nuclides which have few-second half-lives and have been assigned to
Rf were obtained together with those of spontaneously fissioning nuclides which have few-second half-lives and have been assigned to 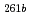 Rf and a longer-lived state of
Rf and a longer-lived state of  Rf. The excitation function of few-second spontaneously fissioning nuclide exhibited the maximum cross section at the
Rf. The excitation function of few-second spontaneously fissioning nuclide exhibited the maximum cross section at the  O beam energy of 94.8 MeV. The shape of the excitation function was almost the same as that of
O beam energy of 94.8 MeV. The shape of the excitation function was almost the same as that of 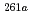 Rf, whereas it was quite different from those of
Rf, whereas it was quite different from those of 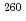 Rf and
Rf and  Rf. A few-second spontaneously fissioning nuclide previously reported as
Rf. A few-second spontaneously fissioning nuclide previously reported as 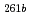 Rf and
Rf and  Rf observed in
Rf observed in Cm +
Cm +  O reaction was identified as
O reaction was identified as 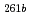 Rf.
Rf.
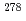 113 of the 113th element
113 of the 113th elementMorita, Kosuke*; Morimoto, Koji*; Kaji, Daiya*; Haba, Hiromitsu*; Ozeki, Kazutaka*; Kudo, Yuki*; Sumita, Takayuki*; Wakabayashi, Yasuo*; Yoneda, Akira*; Tanaka, Kengo*; et al.
Journal of the Physical Society of Japan, 81(10), p.103201_1 - 103201_4, 2012/10
Times Cited Count:167 Percentile:97.27(Physics, Multidisciplinary)An isotope of the 113th element,  113, was produced in a nuclear reaction with a
113, was produced in a nuclear reaction with a  Zn beam on a
Zn beam on a  Bi target. We observed six consecutive
Bi target. We observed six consecutive  decays following the implantation of a heavy particle in nearly the same position in the semiconductor detector, in extremely low background condition. The fifth and sixth decays are fully consistent with the sequential decays of
decays following the implantation of a heavy particle in nearly the same position in the semiconductor detector, in extremely low background condition. The fifth and sixth decays are fully consistent with the sequential decays of  Db and
Db and  Lr both in decay energies and decay times. This indicates that the present decay chain consisted of
Lr both in decay energies and decay times. This indicates that the present decay chain consisted of 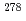 113,
113, 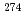 Rg (Z = 111),
Rg (Z = 111), 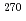 Mt (Z = 109),
Mt (Z = 109), 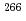 Bh (Z = 107),
Bh (Z = 107),  Db (Z = 105), and
Db (Z = 105), and 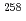 Lr (Z = 103) with firm connections. This result, together with previously reported results from 2004 and 2007, conclusively leads the unambiguous production and identification of the isotope
Lr (Z = 103) with firm connections. This result, together with previously reported results from 2004 and 2007, conclusively leads the unambiguous production and identification of the isotope 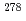 113, of the 113th element.
113, of the 113th element.
 SO
SO /HNO
/HNO mixed solution
mixed solutionLi, Z.*; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Kikuchi, Takahiro; Nagame, Yuichiro; Sch del, M.; Pershina, V.*; et al.
del, M.; Pershina, V.*; et al.
Radiochimica Acta, 100(3), p.157 - 164, 2012/03
Times Cited Count:13 Percentile:69.01(Chemistry, Inorganic & Nuclear)Murayama, Hiroshi*; Goto, Shinichi*; Kudo, Hisaaki*; Tsukada, Kazuaki; Asai, Masato; Toyoshima, Atsushi; Sato, Tetsuya; Nagame, Yuichiro
no journal, ,
no abstracts in English
 SO
SO /HNO
/HNO mixed solution ([H
mixed solution ([H ] = 1.0 M)
] = 1.0 M)Li, Z.; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Kikuchi, Takahiro; Sch del, M.*; Nagame, Yuichiro; Liang, X. H.*; et al.
del, M.*; Nagame, Yuichiro; Liang, X. H.*; et al.
no journal, ,
Cation-exchange behavior of  Rf (T
Rf (T = 78 s) produced in the
= 78 s) produced in the  Cm(
Cm( O, 5n) reaction was studied on a "one-atom-at-a-time" scale in H
O, 5n) reaction was studied on a "one-atom-at-a-time" scale in H SO
SO (0.15-0.69 M)/HNO
(0.15-0.69 M)/HNO mixed solutions ([H
mixed solutions ([H ] = 1.0 M) using an automated ion-exchange separation apparatus coupled with the detection system for alpha-spectroscopy. It was found that adsorption probability (%ads) of
] = 1.0 M) using an automated ion-exchange separation apparatus coupled with the detection system for alpha-spectroscopy. It was found that adsorption probability (%ads) of  Rf on cation-exchange resin decreases with increasing [HSO
Rf on cation-exchange resin decreases with increasing [HSO
 ], showing a successive formation of its sulfate complexes. Rf exhibited much weaker formation of the complexes than the lighter homologues Zr and Hf, which is qualitatively in good agreement with theoretical calculations including relativistic effects.
], showing a successive formation of its sulfate complexes. Rf exhibited much weaker formation of the complexes than the lighter homologues Zr and Hf, which is qualitatively in good agreement with theoretical calculations including relativistic effects.
 Bk and
Bk and 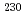 Am
AmKaji, Daiya*; Haba, Hiromitsu*; Kasamatsu, Yoshitaka*; Kudo, Yuki*; Morimoto, Koji*; Morita, Kosuke*; Ozeki, Kazutaka*; Sumita, Takayuki*; Yoneda, Akira*; Koura, Hiroyuki; et al.
no journal, ,
no abstracts in English
Murayama, Hiroshi*; Kojima, Takayuki*; Murakami, Masashi*; Goto, Shinichi*; Kudo, Hisaaki*; Tsukada, Kazuaki; Asai, Masato; Sato, Tetsuya; Sato, Nozomi; Nagame, Yuichiro
no journal, ,
For investigation of chemical properties of superheavy element Rf, we have developed an on-line isothermal gas chromatographic apparatus. We performed to optimize experimental conditions for chemical separation and observe on-line isothermal gas chromatographic behavior of volatile chloride compounds of group-4 elements Zr and Hf. The observed overall efficiency which include transportation from a target chamber to the chemical apparatus, chemical efficiency and transportation from the apparatus to a detection site is about 10%. The isothermal gas chromatographic behavior of Zr and Hf were measured precisely.
 SO
SO /HNO
/HNO mixed solution ([H
mixed solution ([H ] = 1.0 M)
] = 1.0 M)Li, Z.; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Sch del, M.*; Nagame, Yuichiro; Liang, X. H.*; Kasamatsu, Yoshitaka*; et al.
del, M.*; Nagame, Yuichiro; Liang, X. H.*; Kasamatsu, Yoshitaka*; et al.
no journal, ,
Cation-exchange behavior of  Rf (T
Rf (T = 78 s) produced in the
= 78 s) produced in the  Cm(
Cm( O,5n) reaction was studied on a "one-atom-at-a-time" scale in 0.15-0.69 M H
O,5n) reaction was studied on a "one-atom-at-a-time" scale in 0.15-0.69 M H SO
SO /HNO
/HNO mixed solutions ([H
mixed solutions ([H ] = 1.0 M) using an automated ion-exchange separation apparatus coupled with a detection system for alpha-spectroscopy (AIDA). It was found that adsorption probabilities (%ads) of Rf on the cation-exchange resin decrease with increasing [HSO
] = 1.0 M) using an automated ion-exchange separation apparatus coupled with a detection system for alpha-spectroscopy (AIDA). It was found that adsorption probabilities (%ads) of Rf on the cation-exchange resin decrease with increasing [HSO
 ], showing successive formation of Rf sulfate complexes. Rf exhibits a weaker complex formation than the lighter homologues Zr and Hf.
], showing successive formation of Rf sulfate complexes. Rf exhibits a weaker complex formation than the lighter homologues Zr and Hf.
 Cm +
Cm +  O reaction
O reactionMurakami, Masashi*; Goto, Shinichi*; Murayama, Hiroshi*; Kojima, Takayuki*; Kaji, Daiya*; Morimoto, Koji*; Haba, Hiromitsu*; Sumita, Takayuki*; Sakai, Ryutaro*; Kudo, Yuki*; et al.
no journal, ,
Short-lived isomer 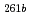 Rf were observed only in the decay chain of
Rf were observed only in the decay chain of 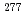 Cn or
Cn or 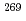 Hs, so far. In this work, we performed to confirm that the
Hs, so far. In this work, we performed to confirm that the 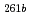 Rf would be produced in the reaction of
Rf would be produced in the reaction of  Cm +
Cm + O simultaneously with the production of
O simultaneously with the production of 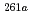 Rf (T
Rf (T = 68s) by using a gas-filled recoil ion separator (GARIS) at the RIKEN linear accelerator.
= 68s) by using a gas-filled recoil ion separator (GARIS) at the RIKEN linear accelerator.
Kojima, Takayuki*; Murayama, Hiroshi*; Murakami, Masashi*; Goto, Shinichi*; Haba, Hiromitsu*; Kaji, Daiya*; Morimoto, Koji*; Kudo, Yuki*; Morita, Kosuke*; Kikunaga, Hidetoshi*; et al.
no journal, ,
In order to study on a gas phase chemistry of rutherfordium (Rf, Z=104) by using a gas-jet system coupled with the GARIS at RIKEN, we performed to optimize experimental conditions of a isothermal gas chromatographic apparatus for chlorides of group-4 elements.