Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hayashi, Hirokazu; Tsubata, Yasuhiro; Sato, Takumi
Nihon Genshiryoku Gakkai Wabun Rombunshi (Internet), 22(3), p.97 - 107, 2023/08
The Japan Atomic Energy Agency has chosen nitride fuel as the first candidate for the transmutation of long-lived minor actinides (MA) using accelerator-driven systems (ADS). The pyrochemical method has been considered for reprocessing spent MA nitride fuels, because their decay heat should be very large for aqueous reprocessing. This study was conducted to investigate the effect of decay heat on the pyrochemical reprocessing of MA nitride fuels. On the basis of the estimated decay heats and the temperature limits of the materials that are to be handled in pyrochemical reprocessing, quantities adequate for handling in argon gas atmosphere were evaluated. From these considerations, we proposed that an electrorefiner with a diameter of 26 cm comprising 12 cadmium (Cd) cathodes with a diameter of 4 cm is suitable. On the basis of the size of the electrorefiner, the number necessary to reprocess spent MA fuels from 1 ADS in 200 days was evaluated to be 25. Furthermore, the amount of Cd-actinides (An) alloy to produce An nitrides by the nitridation-distillation combined reaction process was proposed to be about one-quarter that of Cd-An cathode material. The evaluated sizes and required numbers of equipment support the feasibility of pyrochemical reprocessing for MA nitride fuels.
 (M = Gd, Np) by the reaction of MN with Pd and chlorination of MPd
(M = Gd, Np) by the reaction of MN with Pd and chlorination of MPd using cadmium chloride
using cadmium chlorideHayashi, Hirokazu; Shibata, Hiroki; Sato, Takumi; Otobe, Haruyoshi
Journal of Radioanalytical and Nuclear Chemistry, 332(2), p.503 - 510, 2023/02
Times Cited Count:0 Percentile:0.01(Chemistry, Analytical)The formation of MPd (M = Gd, Np) by the reaction of MN with Pd at 1323 K in Ar gas flow was observed. Cubic AuCu
(M = Gd, Np) by the reaction of MN with Pd at 1323 K in Ar gas flow was observed. Cubic AuCu -type GdPd
-type GdPd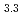 (
( = 0.4081
= 0.4081  0.0001 nm) and NpPd
0.0001 nm) and NpPd (
( = 0.4081
= 0.4081  0.0001 nm) were identified, respectively. The product obtained from the reaction of NpN with Pd contained additional phases including the hexagonal TiNi
0.0001 nm) were identified, respectively. The product obtained from the reaction of NpN with Pd contained additional phases including the hexagonal TiNi -type NpPd
-type NpPd . Chlorination of the MPd
. Chlorination of the MPd (M = Gd, Np) samples was accomplished by the solid-state reaction using cadmium chloride at 673 K in a dynamic vacuum. Pd-rich solid solution phase saturated with Cd and an intermetallic compound PdCd were obtained as by-products of MCl
(M = Gd, Np) samples was accomplished by the solid-state reaction using cadmium chloride at 673 K in a dynamic vacuum. Pd-rich solid solution phase saturated with Cd and an intermetallic compound PdCd were obtained as by-products of MCl formation.
formation.
Sato, Rika*; Nishi, Tsuyoshi*; Ota, Hiromichi*; Hayashi, Hirokazu; Sugawara, Takanori; Nishihara, Kenji
Dai-43-Kai Nihon Netsu Bussei Shimpojiumu Koen Rombunshu (CD-ROM), 3 Pages, 2022/10
no abstracts in English
Murakami, Tsuyoshi*; Hayashi, Hirokazu
Journal of Nuclear Materials, 558, p.153330_1 - 153330_7, 2022/01
Times Cited Count:0 Percentile:0.01(Materials Science, Multidisciplinary)Excess amounts of dissolution agents, CdCl and ZrCl
and ZrCl , are required to dissolve transuranium (TRU: Pu and minor actinides) nitrides into LiCl-KCl melts at the chemical dissolution step, which is the first step in the reprocessing of used nitride fuels. We propose an electrochemical process where the remaining Zr and Cd are recovered from the melts to be recycled as dissolution agents for the chemical dissolution step, leaving TRU in the melts. Since the initial concentration ratio of CdCl
, are required to dissolve transuranium (TRU: Pu and minor actinides) nitrides into LiCl-KCl melts at the chemical dissolution step, which is the first step in the reprocessing of used nitride fuels. We propose an electrochemical process where the remaining Zr and Cd are recovered from the melts to be recycled as dissolution agents for the chemical dissolution step, leaving TRU in the melts. Since the initial concentration ratio of CdCl /ZrCl
/ZrCl remaining in the melts would depend on the condition of the chemical dissolution step and would vary during the proposed electrochemical recovery process, electrochemical behaviors of Zr and Cd were investigated in LiCl-KCl melts with various concentration ratios of CdCl
remaining in the melts would depend on the condition of the chemical dissolution step and would vary during the proposed electrochemical recovery process, electrochemical behaviors of Zr and Cd were investigated in LiCl-KCl melts with various concentration ratios of CdCl /ZrCl
/ZrCl at 723 K to confirm the basic feasibility of the proposed process. Potentiostatic electrolysis was performed using a liquid Cd cathode at -1.05 V (vs. Ag/AgCl), which was a more positive potential than the redox potentials of TRU on the liquid Cd electrode. The obtained results showed that the current efficiency for recovering Zr and Cd from the melts was as high as 100% regardless of the CdCl
at 723 K to confirm the basic feasibility of the proposed process. Potentiostatic electrolysis was performed using a liquid Cd cathode at -1.05 V (vs. Ag/AgCl), which was a more positive potential than the redox potentials of TRU on the liquid Cd electrode. The obtained results showed that the current efficiency for recovering Zr and Cd from the melts was as high as 100% regardless of the CdCl /ZrCl
/ZrCl concentration ratio in the melts.
concentration ratio in the melts.
Shibata, Hiroki; Saito, Hiroaki; Hayashi, Hirokazu; Takano, Masahide
JAEA-Data/Code 2019-023, 138 Pages, 2020/03
Transmutation of minor actinides in the form of nitride fuel by the accelerator driven system has been developed to reduce the radiotoxicity and volume in the radioactive wastes. Nitride fuel behavior under irradiation condition is necessary for its design and development. Nitride fuel performance analysis module based on light water reactor fuel performance code, FEMAXI-7, was developed by introducing fundamental properties of nitride pellet, 9Cr-1Mo ferrite cladding, and Pi-Bi coolant. As a result of test analysis with this module, we have understood that the nitride fuel shows excellent behavior under irradiation due to its high thermal conductivity. We found that, however, it may be a main concern that fuel cladding integrity is maintained during irradiation in which pellet-cladding mechanical interaction is increased by He gas release, low creep rate of nitride pellet at low temperatures, and high creep rate of cladding above 873 K.
Tateno, Haruka; Sato, Takumi; Tsubata, Yasuhiro; Hayashi, Hirokazu
Journal of Nuclear Science and Technology, 57(3), p.224 - 235, 2020/03
Times Cited Count:6 Percentile:55.67(Nuclear Science & Technology)Fuel cycle technology for the transmutation of long-lived minor actinides (MAs) using an accelerator-driven system has been developed using the double-strata fuel cycle concept. A mononitride solid solution of MAs and Pu diluted with ZrN is a prime fuel candidate for the accelerator-driven transmutation of MAs. Pyro-reprocessing is suitable for recycling the residual MAs in irradiated nitride fuel with high radiation doses and decay heat. Spent nitride fuel is anodically dissolved, and the actinides are recovered simultaneously into a liquid cadmium cathode via molten salt electrorefining. The process should be designed to achieve the target recovery yield of MAs and the acceptable impurity level of rare earths in the recovered material. We evaluated the material balance during the pyro-reprocessing of spent nitride fuel to gain important insight on the design process. We examined the effects of changing processing conditions on material flow and quantity of waste.
Hayashi, Hirokazu; Chiba, Rikiya*
Progress in Nuclear Science and Technology (Internet), 5, p.196 - 199, 2018/11
Uranium-free nitride fuel has been chosen as the first candidate for transmutation of long-lived minor actinides (MA: Np, Am, Cm) using sub-critical accelerator-driven system (ADS) under the double strata fuel cycle concept by Japan Atomic Energy Agency (JAEA). Dissolution behavior of ZrN-based nitrides in nitric acid is examined using lanthanides as surrogate materials of TRU elements. Chemical analysis of the ZrN-based lanthanide nitrides dissolved in nitric acid is also carried out.
Hayashi, Hirokazu; Sato, Takumi; Shibata, Hiroki; Tsubata, Yasuhiro
NEA/NSC/R(2017)3, p.427 - 432, 2017/11
Transmutation of long-lived radioactive nuclides including minor actinides (MA: Np, Am, Cm) has been studied in Japan Atomic Energy Agency (JAEA). Pb-Bi cooled sub-critical accelerator-driven system (ADS) is regarded as one of the powerful tools for transmutation of MA under the double strata fuel cycle concept. Uranium-free MA-Pu nitride fuel was chosen as the first candidate for MA transmutation. Reprocessing of spent ADS fuel and reusing MA recovered from the spent ADS fuels is necessary to improve the transmutation ratio. A pyrochemical process has been proposed as the first candidate for reprocessing of the spent nitride fuel for MA transmutation, because this technique has some advantages over aqueous process, such as the resistance to radiation damage, which is an important issue for the fuels containing large amounts of highly radioactive MA, and feasibility for recovering expensive N-15 in the spent fuels to be reused. This paper overviews the current status of the technology development, including our recent study. Development of the anode suitable for electro-refining of nitride fuels and that of the apparatus for renitridation of the metals recovered in Cd cathode for 100g-Cd scale cold tests are main topics. Evaluation of the batch sizes of each process, which is necessary for estimating the scale of the engineering-apparatus, with considering the decay heat of MA and FP, will also be introduced.
 and (U,Zr)O
and (U,Zr)O solid solution using MoCl
solid solution using MoCl
Sato, Takumi; Shibata, Hiroki; Hayashi, Hirokazu; Takano, Masahide; Kurata, Masaki
Journal of Nuclear Science and Technology, 52(10), p.1253 - 1258, 2015/10
Times Cited Count:6 Percentile:45.92(Nuclear Science & Technology)In order to explore the applicability of the chlorination by MoCl as a potential pretreatment technique for waste treatment of fuel debris by pyrochemical methods, chlorination experiments of UO
as a potential pretreatment technique for waste treatment of fuel debris by pyrochemical methods, chlorination experiments of UO and (U
and (U Zr
Zr )O
)O simulated fuel debris were carried out in two steps: the first one is a chlorination reaction by homogeneous heating, the second one is a volatilization of molybdenum by-product by heating under temperature gradient condition. Most of UO
simulated fuel debris were carried out in two steps: the first one is a chlorination reaction by homogeneous heating, the second one is a volatilization of molybdenum by-product by heating under temperature gradient condition. Most of UO and (U
and (U Zr
Zr )O
)O powder were converted to UCl
powder were converted to UCl or UCl
or UCl and ZrCl
and ZrCl mixture at 573 K, respectively. In the case of (U
mixture at 573 K, respectively. In the case of (U Zr
Zr )O
)O sintered particle, most of sample was converted to the chlorides because the products evaporated and be separated from sample surface at 773 K, while only the surface of the sample disk was converted to the chlorides at 573 and 673 K. Most of molybdenum by-product and ZrCl
sintered particle, most of sample was converted to the chlorides because the products evaporated and be separated from sample surface at 773 K, while only the surface of the sample disk was converted to the chlorides at 573 and 673 K. Most of molybdenum by-product and ZrCl were separated from UCl
were separated from UCl by volatilization at 573 K.
by volatilization at 573 K.
Tsujimoto, Kazufumi; Sasa, Toshinobu; Maekawa, Fujio; Matsumura, Tatsuro; Hayashi, Hirokazu; Kurata, Masaki; Morita, Yasuji; Oigawa, Hiroyuki
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.657 - 663, 2015/09
To continue the utilization of the nuclear fission energy, the management of the high-level radioactive waste is one of the most important issues to be solved. Partitioning and Transmutation technology of HLW is expected to be effective to mitigate the burden of the HLW disposal by reducing the radiological toxicity and heat generation. The Japan Atomic Energy Agency (JAEA) has been conducting the research and development on accelerator-driven subcritical system (ADS) as a dedicated system for the transmutation of long-lived radioactive nuclides. This paper overviews the recent progress and future R&D plan of the study on the ADS and related fuel cycle technology in JAEA.
Hayashi, Hirokazu; Nishi, Tsuyoshi*; Sato, Takumi; Kurata, Masaki
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.1811 - 1817, 2015/09
Transmutation of long-lived radioactive nuclides including minor actinides (MA: Np, Am, Cm) has been studied in Japan Atomic Energy Agency (JAEA). Accelerator-driven system (ADS) is regarded as one of the powerful tools for transmutation of MA under the double strata fuel cycle concept. Uranium-free nitride fuel was chosen as the first candidate fuel for MA transmutation using ADS. To improve the transmutation ratio of MA, reprocessing of spent fuel and reusing MA recovered from the spent fuels is necessary. Our target is to transmute 99% of MA arisen from commercial power reactor fuel cycle, with which the period until the radiotoxicity drops below that of natural uranium can be shorten from about 5000 years to about 300 years. A pyrochemical process has been proposed as the first candidate for reprocessing of the spent nitride fuel. This paper overviews the current status of the nitride fuel cycle technology. Our recent study on fuel fabrication, fuel property measurements, reprocessing of spent fuel, development of the property database of MA nitride fuel, and fuel behavior simulation code are introduced. Our research and development (R&D) plan based on the roadmap of the development is also introduced.
Shibata, Hiroki; Hayashi, Hirokazu; Koyama, Tadafumi*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 83(7), p.532 - 536, 2015/07
Times Cited Count:1 Percentile:1.67(Electrochemistry)The electrochemical properties of curium in a LiCl-KCl eutectic melt were studied in the temperature range of 718-823 K. A small electrochemical cell used in this study was designed for the electrochemical measurement with a small amount (1-20 mg) of the highly radioactive minor actinides contained in molten salts achieved in a hot cell. Our data of apparent standard potentials of a Cm /Cm couple are reasonably in agreement with Osipenko's data (2011) and are lower than Martinot's data (1975). The validity of our data and the reported apparent standard potentials were discussed.
/Cm couple are reasonably in agreement with Osipenko's data (2011) and are lower than Martinot's data (1975). The validity of our data and the reported apparent standard potentials were discussed.
Hayashi, Hirokazu; Nishi, Tsuyoshi; Takano, Masahide; Sato, Takumi; Shibata, Hiroki; Kurata, Masaki
NEA/NSC/R(2015)2 (Internet), p.360 - 367, 2015/06
Uranium-free nitride fuel was chosen as the first candidate for transmutation of long-lived minor actinides (MA) using accelerator-driven system (ADS) under the double strata fuel cycle concept by Japan Atomic Energy Agency (JAEA). The advantages of nitride fuel are good thermal properties and large mutual solubility among actinide elements. A pyrochemical process is proposed as the first candidate for the reprocessing of the spent nitride fuel, because this technique has some advantages over aqueous process, such as the resistance to radiation damage, which is an important issue for the fuels containing large amounts of highly radioactive MA. This paper overviews the recent progress and future R&D plan of the study on the nitride fuel cycle technology in JAEA.
Hayashi, Hirokazu; Akabori, Mitsuo; Minato, Kazuo
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1331 - 1334, 2015/02
Times Cited Count:0 Percentile:0.01(Chemistry, Analytical)Electrochemical behavior of Am in NaCl-2CsCl melt at 823 K was investigated by transient electrochemical techniques such as cyclic voltammetry and differential pulse voltammetry. The results show that Am(III) ion is reduced to Am metal by a two-step mechanism via Am(II) ion. Formal standard potential of Am(III)/Am(II) and that of Am(II)/Am(0) redox couples have been determined to be -2.73 and -2.97 V vs Cl /Cl
/Cl , respectively.
, respectively.
Hayashi, Hirokazu; Sato, Takumi; Shibata, Hiroki; Kurata, Masaki; Iwai, Takashi; Arai, Yasuo
Science China; Chemistry, 57(11), p.1427 - 1431, 2014/11
Times Cited Count:5 Percentile:5.84(Chemistry, Multidisciplinary)Nitride fuels have several advantages, such as high thermal conductivity and high metal density like metallic fuels, and high melting point and isotropic crystal structure like oxide fuels. Since the late 1990s, the partitioning and transmutation of minor actinides (MA) has been studied to decrease the long term radio-toxicity of high level waste and mitigate the burden on the final disposal. Japan Atomic Energy Agency (JAEA) has been proposing dedicated transmutation cycle using the Accelerator-Driven System (ADS) with the nitride fuels containing MA. We have been developing the nitride fuel cycle including pyrochemical process. Our focus is on electrolysis of nitride fuels and refabrication of nitride fuel from the recovered actinides because other processes are similar to the technology for the metal fuel treatment and have been studied elsewhere. In this paper, we summarized our activity on developments of the pyrochemical treatment of the spent nitride fuels.
Shibata, Hiroki; Hayashi, Hirokazu; Akabori, Mitsuo; Arai, Yasuo; Kurata, Masaki
Journal of Physics and Chemistry of Solids, 75(8), p.972 - 976, 2014/08
Times Cited Count:18 Percentile:59.26(Chemistry, Multidisciplinary)Gibbs free energies of formation of six Ce-Cd intermetallic compounds, CeCd, CeCd , CeCd
, CeCd , CeCd
, CeCd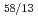 , CeCd
, CeCd and CeCd
and CeCd , were evaluated systematically using electrochemical techniques in the temperature range from 673 to 923 K in the LiCl-KCl-CeCl
, were evaluated systematically using electrochemical techniques in the temperature range from 673 to 923 K in the LiCl-KCl-CeCl -CdCl
-CdCl molten salt bath. The linear dependence of the Gibbs free energies of formation on temperature yields to the enthalpies and entropies of formation of these intermetallic compounds. By extrapolating the molar Gibbs free energy of Ce-Cd intermetallic compounds to the Cd distillation temperature, it was clear that the molar Gibbs free energy of Ce in Ce-Cd intermetallic compounds decreases gradually from CeCd
molten salt bath. The linear dependence of the Gibbs free energies of formation on temperature yields to the enthalpies and entropies of formation of these intermetallic compounds. By extrapolating the molar Gibbs free energy of Ce-Cd intermetallic compounds to the Cd distillation temperature, it was clear that the molar Gibbs free energy of Ce in Ce-Cd intermetallic compounds decreases gradually from CeCd to CeCd
to CeCd and attains to the minimum value at CeCd
and attains to the minimum value at CeCd . This suggests on the Cd distillation from the U-Pu-Ce-Cd alloy that the dissolution of U or Pu into CeCd
. This suggests on the Cd distillation from the U-Pu-Ce-Cd alloy that the dissolution of U or Pu into CeCd should be mostly taken into consideration.
should be mostly taken into consideration.
Takano, Masahide; Hayashi, Hirokazu; Minato, Kazuo
Journal of Nuclear Materials, 448(1-3), p.66 - 71, 2014/05
Times Cited Count:2 Percentile:16.44(Materials Science, Multidisciplinary)A powder sample of curium nitride (CmN) containing 0.35%-PuN and 3.59%-AmN was prepared by carbothermic nitridation of the oxide. The lattice expansion induced by self-irradiation damage at room temperature was measured as a function of time. The saturated  a/a
a/a value was 0.43%, which is greater than those for transuranium dioxides available in literature. The undamaged lattice parameter at 297
value was 0.43%, which is greater than those for transuranium dioxides available in literature. The undamaged lattice parameter at 297 1 K was determined to be 0.50261
1 K was determined to be 0.50261 0.00006 nm. Temperature dependence of the lattice parameter was measured by a high temperature X-ray diffractometer in the temperature range up to 1375 K. The linear thermal expansion of the lattice from 293 to 1273 K is 0.964% and the corresponding thermal expansion coefficient is 9.84
0.00006 nm. Temperature dependence of the lattice parameter was measured by a high temperature X-ray diffractometer in the temperature range up to 1375 K. The linear thermal expansion of the lattice from 293 to 1273 K is 0.964% and the corresponding thermal expansion coefficient is 9.84  10
10 K
K . Comparing with the other actinide nitrides, it was found that CmN lies between the higher expansion nitrides (PuN and AmN) and the lower expansion nitrides (UN and NpN).
. Comparing with the other actinide nitrides, it was found that CmN lies between the higher expansion nitrides (PuN and AmN) and the lower expansion nitrides (UN and NpN).
Hayashi, Hirokazu; Takano, Masahide; Kurata, Masaki; Minato, Kazuo
Journal of Nuclear Materials, 440(1-3), p.477 - 479, 2013/09
Times Cited Count:5 Percentile:38.62(Materials Science, Multidisciplinary)Neptunium trichloride of high purity was synthesized by the solid-state reaction of neptunium nitride, which was prepared from the oxide by the carbothermic reduction method, and cadmium chloride in a similar manner as reported for synthesis of AmCl . Lattice parameters of hexagonal NpCl
. Lattice parameters of hexagonal NpCl were determined from the X-ray diffraction pattern to be a = 0.7421
were determined from the X-ray diffraction pattern to be a = 0.7421  0.0006 nm and c = 0.4268
0.0006 nm and c = 0.4268  0.0003 nm, which fairly agree with the reported values (a = 0.742
0.0003 nm, which fairly agree with the reported values (a = 0.742  0.001 nm and c = 0.4281
0.001 nm and c = 0.4281  0.0005 nm). Melting temperature of NpCl
0.0005 nm). Melting temperature of NpCl was measured with about 1 mg of the sample which was hermetically encapsulated in a gold crucible using a differential thermal analyzer with heating and cooling rate of 10 K/min in an argon gas flow (50 mL/min). The melting temperature of NpCl
was measured with about 1 mg of the sample which was hermetically encapsulated in a gold crucible using a differential thermal analyzer with heating and cooling rate of 10 K/min in an argon gas flow (50 mL/min). The melting temperature of NpCl was determined to 1070
was determined to 1070  3 K, which is close to the recommended value 1075
3 K, which is close to the recommended value 1075 30 K, which was derived from the mean value of the melting temperature for UCl
30 K, which was derived from the mean value of the melting temperature for UCl (1115K) and that for PuCl
(1115K) and that for PuCl (1041 K).
(1041 K).
Hayashi, Hirokazu; Takano, Masahide; Otobe, Haruyoshi; Koyama, Tadafumi*
Journal of Radioanalytical and Nuclear Chemistry, 297(1), p.139 - 144, 2013/07
Times Cited Count:2 Percentile:18.63(Chemistry, Analytical)Curium trichloride was synthesized by the solid state reaction of curium nitride with cadmium chloride heated from room temperature to 748K in a dynamic vacuum. The product was hexagonal  CmCl
CmCl , of which lattice parameters were determined to be a= 0.7385
, of which lattice parameters were determined to be a= 0.7385 0.0005 and c= 0.4201
0.0005 and c= 0.4201 0.0005 nm. The melting temperature of the
0.0005 nm. The melting temperature of the  CmCl
CmCl sample was determined to be 970
sample was determined to be 970 3 K by differential thermal analyses using a gold crucible. These values are close to those reported in literatures. The results show that mg-scale CmCl
3 K by differential thermal analyses using a gold crucible. These values are close to those reported in literatures. The results show that mg-scale CmCl samples for thermochemical measurements were prepared from the purified oxide sample without the use of corrosive reagents.
samples for thermochemical measurements were prepared from the purified oxide sample without the use of corrosive reagents.
Hayashi, Hirokazu; Hagiya, Hiromichi; Kim, S.-Y.*; Morita, Yasuji; Akabori, Mitsuo; Minato, Kazuo
Journal of Radioanalytical and Nuclear Chemistry, 296(3), p.1275 - 1286, 2013/06
Times Cited Count:4 Percentile:32.48(Chemistry, Analytical) Cm was separated and recovered as an oxalate from
Cm was separated and recovered as an oxalate from  Cm-
Cm- Pu mixed oxide which had been
Pu mixed oxide which had been  Cm oxide sample prepared 40 years ago. Plutonium ions were removed from the solution prepared by dissolution of
Cm oxide sample prepared 40 years ago. Plutonium ions were removed from the solution prepared by dissolution of  Cm-
Cm- Pu mixed oxide in nitric acid, by using an anion exchange resin column. Curium oxalate, a precursor compound of curium oxide, was prepared from the purified curium solution and supplied for the syntheses and measurements of the thermochemical properties of curium compounds.
Pu mixed oxide in nitric acid, by using an anion exchange resin column. Curium oxalate, a precursor compound of curium oxide, was prepared from the purified curium solution and supplied for the syntheses and measurements of the thermochemical properties of curium compounds.