Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Abe, Hiroshi*; Nemoto, Fumiya*; Hiroi, Kosuke; Oishi, Kazuki*; Takata, Shinichi
Journal of Molecular Liquids, 346, p.117035_1 - 117035_6, 2022/01
Times Cited Count:4 Percentile:61.27(Chemistry, Physical)Nemoto, Fumiya*; Kofu, Maiko; Nagao, Michihiro*; Oishi, Kazuki*; Takata, Shinichi; Suzuki, Junichi*; Yamada, Takeshi*; Shibata, Kaoru; Ueki, Takeshi*; Kitazawa, Yuzo*; et al.
Journal of Chemical Physics, 149(5), p.054502_1 - 054502_11, 2018/08
Times Cited Count:19 Percentile:70.02(Chemistry, Physical)Kanayama, Fumihiko; Hagiya, Kazuaki; Sunaoshi, Mizuho; Muraguchi, Yoshinori; Satomi, Shinichi; Nemoto, Koichi; Terunuma, Akihiro; Shiraishi, Kunio; Ito, Shinichi
JAEA-Technology 2011-011, 36 Pages, 2011/06
Dismantling activities of equipments in JAERI's Reprocessing Test Facility (JRTF) started from 1996 as a part of decommissioning of this facility. Removing out of the large liquid waste storage tank LV-2 as a whole tank without cutting in pieces from the annex building B to confirm safety and efficiency of this method started from 2006. After preparatory works, ceiling of LV-2 room was opened, and LV-2 was transferred. Useful data were collected on manpower, radiation control and waste amount through the preparatory works, and work efficiency was analyzed by use of these data.
Terunuma, Akihiro; Naito, Akira; Nemoto, Koichi; Usami, Jun; Tomii, Hiroyuki; Shiraishi, Kunio; Ito, Shinichi
JAEA-Review 2010-038, 96 Pages, 2010/09
Japan Atomic Energy Agency (JAEA) has midterm plan for decommissioning the facilities being finished their role and the facilities that became unnecessary by shifting their functions to other facilities. In the first midterm plan (from the latter half of fiscal year 2005 to fiscal year 2009), decommissioning of five facilities (Ceramic Research Facility, Plutonium Research Facility No.2, Metallurgy Research Facility, Isotope Separation Research Facility and Reprocessing Test Facility) had been carried out in order to release controlled area and dismantle the facilities in Nuclear Science Research Institute (NSRI), JAEA. The decommissioning activity for each facility had been reported to the regulatory body and municipalities. On this report, we summarize the each activity for five facilities by reviewing the reports to the regulatory body and municipalities. We also added the knowledge obtained through the activity.
; Miyachi, Shigehiko; ;
Proceedings of 10th International Conference on Nuclear Engineering (ICONE-10), 0 Pages, 2002/00
None
; ; ;
Solvent Extraction and Ion Exchange, 16(6), p.1357 - 1367, 2002/00
Times Cited Count:52 Percentile:84.27(Chemistry, Multidisciplinary)None
; ; ;
Journal of Nuclear Science and Technology, 35(2), p.130 - 136, 1998/02
None
Koma, Yoshikazu; Watanabe, Masayuki; Nemoto, Shinichi; Ozawa, Masaki; Okamoto, Fumitoshi; Tanaka, Yasumasa
PNC TN8410 95-193, 49 Pages, 1995/06
None
Shibata, Atsuhiro; P.Y.COR*; *; ; ; *; Tanaka, Yasumasa
PNC TN8410 95-112, 100 Pages, 1995/05
PNC has developed the TRUEX process to recover transuranium elements from high level liquid waste. This process uses CMPO as extracting solvent. DIAMEX process which uses Diamide has been developed bY CEA in France. The aim of basic studies was to get some comparison data between CMPO and Diamide. We have conducted the experiments regarding properties of Ln(III) extraction and behavior of third phase formation. Also, we have investigated properties of Ln(III) extraction with TBP and DBBP to compare bidentate ligand with monodentate ligand. This study was conducted as a frame of PNC/CEA collaboration on minor Actinide partitioning. The conclusions drawn from our studies can be summarized as follows; (1)Trivalent Lanthanides extraction reaction. CMPO, TBP and DBBP ; Ln + 3NO
+ 3NO + 3Extractant
+ 3Extractant 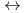 Ln(NO
Ln(NO )
)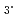 3Extractant. Diamide; Ln
3Extractant. Diamide; Ln + 3NO
+ 3NO + m Diamide
+ m Diamide 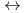 Ln(NO
Ln(NO )
)
 m Diamide : m=O.7
m Diamide : m=O.7 1.8. (2)Extraction equilibrium constant. Apparent extraction equilibrium constants were calculated from the trivalent Lanthanides extraction reaction. The value of the constants were CMPO
1.8. (2)Extraction equilibrium constant. Apparent extraction equilibrium constants were calculated from the trivalent Lanthanides extraction reaction. The value of the constants were CMPO  Diamide
Diamide 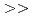 DBBP
DBBP  TBP.CMPO has high extraction efficiency for trivalent metals such as Am, but the back extraction is difficult in nitric acid media. Diamide indicated better behavior about both extraction and back extraction. (3)Separation Factors. We got separation factors (SFs) for intra-group in nitric acid. So SFs for CMPO and Diamide were nearly one, that these solvents could not separate each Lanthanides elements. The other hand, SFs for TBP and DBBP were 2
TBP.CMPO has high extraction efficiency for trivalent metals such as Am, but the back extraction is difficult in nitric acid media. Diamide indicated better behavior about both extraction and back extraction. (3)Separation Factors. We got separation factors (SFs) for intra-group in nitric acid. So SFs for CMPO and Diamide were nearly one, that these solvents could not separate each Lanthanides elements. The other hand, SFs for TBP and DBBP were 2  10. (4)Behavior of third phase formation. When using CMPO or Diamide, even in the case of extraction of nitric acid, third phase formation was found. When third phase was formed, the nitric acid concentration in aqueous phase was 6 N for CMPO, 4 N for Diamide. If Diamide diluted with Decaline was used, third phase wasn't found ...
10. (4)Behavior of third phase formation. When using CMPO or Diamide, even in the case of extraction of nitric acid, third phase formation was found. When third phase was formed, the nitric acid concentration in aqueous phase was 6 N for CMPO, 4 N for Diamide. If Diamide diluted with Decaline was used, third phase wasn't found ...
; Sakai, Toshiyuki*; Sanyoshi, Hirotaka; Iwasaki, Isao*; Kuribayashi, Masakazu*;
PNC TN8410 95-056, 65 Pages, 1995/03
None