Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Eichler, R.*; Asai, Masato; Brand, H.*; Chiera, N. M.*; Di Nitto, A.*; Dressler, R.*; D llmann, Ch. E.*; Even, J.*; Fangli, F.*; Goetz, M.*; et al.
llmann, Ch. E.*; Even, J.*; Fangli, F.*; Goetz, M.*; et al.
EPJ Web of Conferences, 131, p.07005_1 - 07005_7, 2016/12
Times Cited Count:3 Percentile:72.98(Chemistry, Inorganic & Nuclear)In recent years gas-phase chemical studies assisted by physical pre-separation allowed for the productions and investigations of fragile single molecular species of superheavy elements. The latest highlight is the formation of very volatile hexacarbonyl compound of element 106, Sg(CO) . Following this success, second-generation experiments were performed to measure the first bond dissociation energy between the central metal atom and the surrounding ligand. The method using a tubular decomposition reactor was developed and successfully applied to short-lived Mo(CO)
. Following this success, second-generation experiments were performed to measure the first bond dissociation energy between the central metal atom and the surrounding ligand. The method using a tubular decomposition reactor was developed and successfully applied to short-lived Mo(CO) , W(CO)
, W(CO) , and Sg(CO)
, and Sg(CO) .
.
 and W(CO)
and W(CO)
Usoltsev, I.*; Eichler, R.*; Wang, Y.*; Even, J.*; Yakushev, A.*; Haba, Hiromitsu*; Asai, Masato; Brand, H.*; Di Nitto, A.*; D llmann, Ch. E.*; et al.
llmann, Ch. E.*; et al.
Radiochimica Acta, 104(3), p.141 - 151, 2016/03
Times Cited Count:31 Percentile:95.03(Chemistry, Inorganic & Nuclear)Conditions of the production and decomposition of hexacarbonyl complexes of short-lived Mo and W isotopes were investigated to study thermal stability of the heaviest group 6 hexacarbonyl complex Sg(CO) . A tubular flow reactor was tested to decompose the hexacarbonyl complexes and to extract the first bond dissociation energies. A silver was found to be the most appropriate reaction surface to study the decomposition of the group 6 hexacarbonyl. It was found that the surface temperature at which the decomposition occurred was correlated to the first bond dissociation energy of Mo(CO)
. A tubular flow reactor was tested to decompose the hexacarbonyl complexes and to extract the first bond dissociation energies. A silver was found to be the most appropriate reaction surface to study the decomposition of the group 6 hexacarbonyl. It was found that the surface temperature at which the decomposition occurred was correlated to the first bond dissociation energy of Mo(CO) and W(CO)
and W(CO) , indicating that the first bond dissociation energy of Sg(CO)
, indicating that the first bond dissociation energy of Sg(CO) could be determined with this technique.
could be determined with this technique.
Even, J.*; Ackermann, D.*; Asai, Masato; Block, M.*; Brand, H.*; Di Nitto, A.*; D llmann, Ch. E.*; Eichler, R.*; Fan, F.*; Haba, Hiromitsu*; et al.
llmann, Ch. E.*; Eichler, R.*; Fan, F.*; Haba, Hiromitsu*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(3), p.2457 - 2466, 2015/03
Times Cited Count:15 Percentile:77.56(Chemistry, Analytical)Rapid In situ synthesis of metal carbonyl complexes has been demonstrated using short-lived isotopes produced in nuclear fission and fusion reactions. The short-lived isotopes with high recoil energy directly react with carbon-monoxides and form carbonyl complexes. Only highly volatile complexes were fast transported in a gas stream to counting and chemistry devices. Short-lived Mo, Tc, Ru, Rh, W, Re, Os, and Ir were found to form volatile carbonyl complexes, while no volataile complex of Hf and Ta were detected. This technique has been applied to a chemical investigation of the superheavy element Sg (atomic number 106), and will be applicable to various fields of nuclear science with short-lived transition metal isotopes.
Even, J.*; Yakushev, A.*; D llmann, Ch. E.*; Haba, Hiromitsu*; Asai, Masato; Sato, Tetsuya; Brand, H.*; Di Nitto, A.*; Eichler, R.*; Fan, F. L.*; et al.
llmann, Ch. E.*; Haba, Hiromitsu*; Asai, Masato; Sato, Tetsuya; Brand, H.*; Di Nitto, A.*; Eichler, R.*; Fan, F. L.*; et al.
Science, 345(6203), p.1491 - 1493, 2014/09
Times Cited Count:63 Percentile:83.28(Multidisciplinary Sciences)A new superheavy element complex, a seaborgium carbonyl, has been successfully synthesized, and its adsorption property has been studied using a cryo-thermochromatography and  -detection apparatus COMPACT. Nuclear reaction products of short-lived
-detection apparatus COMPACT. Nuclear reaction products of short-lived  Sg preseparated with a gas-filled recoil ion separator GARIS at RIKEN were directly injected into a gas cell filled with He/CO mixture gas, and chemical reaction products of volatile carbonyl complexes were trasported to COMPACT. The Sg carbonyl complex detected with COMPACT was found to be very volatile with adsorption enthalpy of
Sg preseparated with a gas-filled recoil ion separator GARIS at RIKEN were directly injected into a gas cell filled with He/CO mixture gas, and chemical reaction products of volatile carbonyl complexes were trasported to COMPACT. The Sg carbonyl complex detected with COMPACT was found to be very volatile with adsorption enthalpy of 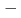 50 kJ/mol, from which we have concluded that this complex should be a Sg hexacarbonyl Sg(CO)
50 kJ/mol, from which we have concluded that this complex should be a Sg hexacarbonyl Sg(CO) . This is the first synthesis of organometallic compounds of transactinide elements for which only simple inorganic comounds have been synthesized so far.
. This is the first synthesis of organometallic compounds of transactinide elements for which only simple inorganic comounds have been synthesized so far.
Abe, Hiroshi; Aone, Shigeo*; Morimoto, Ryo*; Uchida, Hirohisa*
Journal of Alloys and Compounds, 580(Suppl.1), p.S219 - S221, 2013/12
Times Cited Count:5 Percentile:31.8(Chemistry, Physical)no abstracts in English
Abe, Hiroshi; Aone, Shigeo*; Morimoto, Ryo*; Uchida, Hirohisa*; Oshima, Takeshi
Transactions of the Materials Research Society of Japan, 36(1), p.133 - 135, 2011/03
The introduction of vacancies in Pd was found to be effective for an increase in the initial hydrogen absorption rate in a previous study. Also, it was reported that the initial hydrogen absorption rate depends strongly on the surface conditions of metals. Heavy ions with keV ranges can create severe damage and high densities of vacancy near the surface of materials. As well known, the formation of hydride phases can be facilitated by the presence of vacancy because vacancy acts as hydrogen trapping site to form hydrides. Thus, the hydrogen absorption characteristics of Pd may be improved by the irradiation of heavy ions. As a result, the initial hydrogen absorption rate increased due to ion irradiation, and the value became 3 10 times higher than un-irradiated Pd.
10 times higher than un-irradiated Pd.
Yoneda, Yasuhiro; Tamura, Kazuhisa; Abe, Hiroshi; Oshima, Takeshi; Morimoto, Ryo*; Uchida, Hirohisa*; Mizuki, Junichiro
Transactions of the Materials Research Society of Japan, 33(4), p.1053 - 1056, 2008/12
The effect of ion irradiation on palladium (Pd) metal was investigated by hydrogen-absorption measurements, SEM microscopy and synchrotron X-ray diffraction. N irradiation was made with an acceleration energy 350 keV. The initial hydrogen absorption rate of the irradiated Pd was three times larger than that of non-irradiated Pd. The microscopic structure was investigated by using the pair-distribution function (PDF) obtained by X-ray diffraction. Although the average structure of the Pd was f.c.c, the Pd atoms displaced and two occupancy sites are revealed. This site occupancy is closely related with the hydrogen-absorption rate.
irradiation was made with an acceleration energy 350 keV. The initial hydrogen absorption rate of the irradiated Pd was three times larger than that of non-irradiated Pd. The microscopic structure was investigated by using the pair-distribution function (PDF) obtained by X-ray diffraction. Although the average structure of the Pd was f.c.c, the Pd atoms displaced and two occupancy sites are revealed. This site occupancy is closely related with the hydrogen-absorption rate.
Abe, Hiroshi; Morimoto, Ryo*; Sato, Fumiatsu*; Azuma, Yorito*; Uchida, Hirohisa*
Journal of Alloys and Compounds, 408-412, p.348 - 350, 2006/02
Times Cited Count:10 Percentile:54.65(Chemistry, Physical)Mm(misch metal) based hydrogen storage alloys are applied to the negative electrode of the Ni-H battery and other hydrogen storage systems. In such pratical applications, various surface processes of hydrogen molecules often become rate contolling. Therefore, the improvement of the alloy surface is of great importance to promote the initial activation and hydriding rate. We have reported that the alkaline pretreatment of an alloy surfece exhibits a high durability against CO attack. Since low energy ion irradiation is quite useful for surface modification of materials, the hydriding proerties of a Mm is expected on electrochemical hydriding rate of the alloy. As a result, the ion irradiation Mm was found to induce a higher hydriding rate than that of the un-irradiation one.
Abe, Hiroshi; Morimoto, Ryo*; Sato, Fumiatsu*; Azuma, Yorito*; Uchida, Hirohisa*
Journal of Alloys and Compounds, 404-406, p.288 - 292, 2005/12
Times Cited Count:7 Percentile:51.67(Chemistry, Physical)The effect of ion irradiation on the rate of electrochemical hydriding rate of palladium (Pd) was investigated. In this study, ion irradiation onto the Pd surface was made with H , He
, He , Ar
, Ar and N
and N in the acceleration energy range from 30 to 350 keV, and in the ion dose up to 1
in the acceleration energy range from 30 to 350 keV, and in the ion dose up to 1  10
10 cm
cm . As the ion dose was increased, the initial rate of hydriding of Pd was increased. The ion irradiatiion treatment of the surface of a metal induces high concentrations of vacancy. The increased hydriding rate may be caused by the induction of high concentration of vacancy whichi traps hydrogen atoms, and this seems to accelerate the rates of hydride nucleation and growth in the surface. The ion irradiation was found as an effective way to enhance the rate of the initial activation of Pd in the electrochemical hydriding process.
. As the ion dose was increased, the initial rate of hydriding of Pd was increased. The ion irradiatiion treatment of the surface of a metal induces high concentrations of vacancy. The increased hydriding rate may be caused by the induction of high concentration of vacancy whichi traps hydrogen atoms, and this seems to accelerate the rates of hydride nucleation and growth in the surface. The ion irradiation was found as an effective way to enhance the rate of the initial activation of Pd in the electrochemical hydriding process.
Wakabayashi, Go*; Morimoto, Ryo*; Uchida, Hirohisa*; Abe, Hiroshi
no journal, ,
no abstracts in English
Abe, Hiroshi; Uchida, Hirohisa*; Morimoto, Ryo*; Ito, Hisayoshi
no journal, ,
no abstracts in English
Abe, Hiroshi; Uchida, Hirohisa*; Morimoto, Ryo*; Yoneda, Yasuhiro; Mizuki, Junichiro; Ito, Hisayoshi
no journal, ,
no abstracts in English
Aone, Shigeo*; Morimoto, Ryo*; Abe, Hiroshi; Uchida, Hirohisa*
no journal, ,
no abstracts in English
Abe, Hiroshi; Morimoto, Ryo*; Aone, Shigeo*; Uchida, Hirohisa*; Oshima, Takeshi
no journal, ,
no abstracts in English
Abe, Hiroshi; Morimoto, Ryo*; Aone, Shigeo*; Uchida, Hirohisa*; Oshima, Takeshi
no journal, ,
no abstracts in English
Abe, Hiroshi; Morimoto, Ryo*; Aone, Shigeo*; Uchida, Hirohisa*; Oshima, Takeshi
no journal, ,
In previous studies, the induction of vacancy in palladium (Pd) was found to be effective for an increase in the initial hydrogen absorption rate. As well known, the initial hydrogen absorption rate depends strongly on the surface conditions of metals. Based on these results, we made surface modifications of Pd using various ions at TIARA, JAEA. In this study, we perform Cr ion irradiation into Pd, and the hydrogen absorption rate of ion irradiation Pd was evaluated. The Pd irradiated at a fluence to 1 10
10 cm
cm exhibited the highest rate in all samples, and the hydrogen absorption rate became five times higher than that of un-irradiated Pd. Since vacancy type defects which might act as hydrogen trapping site are introduced by irradiation the enhancement of the hydrogen absorption rate obtained inthi study can be interpreted in terms of an increase of hydrogen trapping saites near the surface region due to irradiation of Cr ion into Pd.
exhibited the highest rate in all samples, and the hydrogen absorption rate became five times higher than that of un-irradiated Pd. Since vacancy type defects which might act as hydrogen trapping site are introduced by irradiation the enhancement of the hydrogen absorption rate obtained inthi study can be interpreted in terms of an increase of hydrogen trapping saites near the surface region due to irradiation of Cr ion into Pd.
Yoneda, Yasuhiro; Abe, Hiroshi; Oshima, Takeshi; Morimoto, Ryo*; Uchida, Hirohisa*
no journal, ,
We performed structure analysis of Mg-Fe alloy system prepared by mechanical alloying. For Mg concentrations up to about 15 mol%, mechanical alloying can produce single-phase bcc alloys. By using the conventional average structure analysis and X-ray pair-distribution function method, we can bridge the long-range and short-range order structure of Mg-Fe alloys. The substituted Mg atoms arranged randomly in the low-Mg composition, but Mg atoms came to have the order structure as the Mg composition increases.
Abe, Hiroshi; Morimoto, Ryo*; Uchida, Hirohisa*; Oshima, Takeshi
no journal, ,
Regarding the hydrogen storage in metals, it was reported that the absorption concentration of hydrogen atoms and the hydrogen absorption rate depend strongly on the surface state of metals. For the surface modification of materials, ion implantaton/irradiation with both lon and high energy are known to be a quite useful method. These facts give the possibility that the hydrogen absorptivity in Pd is improved by surface modification using ion irradiation.
Abe, Hiroshi; Aone, Shigeo*; Morimoto, Ryo*; Uchida, Hirohisa*; Oshima, Takeshi
no journal, ,
no abstracts in English