Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sano, Yuichi; Sakamoto, Atsushi; Miyazaki, Yasunori; Watanabe, So; Morita, Keisuke; Emori, Tatsuya; Ban, Yasutoshi; Arai, Tsuyoshi*; Nakatani, Kiyoharu*; Matsuura, Haruaki*; et al.
Proceedings of International Conference on Nuclear Fuel Cycle; Sustainable Energy Beyond the Pandemic (GLOBAL 2022) (Internet), 4 Pages, 2022/07
We developed a hybrid MA(III) recovery process combining MA(III)+Ln(III) co-recovery flowsheet by solvent extraction with TBP and MA(III)/Ln(III) separation flowsheet by simulated moving bed chromatography using HONTA impregnated adsorbents with large particle size porous silica support.
Sasaki, Yuji; Morita, Keisuke; Matsumiya, Masahiko*; Ono, Ryoma*; Shiroishi, Hidenobu*
JOM, 73(4), p.1037 - 1043, 2021/04
Times Cited Count:4 Percentile:34.69(Materials Science, Multidisciplinary)The separation of Dy from Nd is studied from the viewpoint of recycling Dy from Nd magnets. Both metals are lanthanide elements, which means their mutual separation is difficult because of their similar chemical behaviors. All lanthanide elements can be extracted easily by using tetradodecyl-diglycolamide (TDdDGA) extractants, and it has a relatively high separation factor (SF) between Dy and Nd (SF over 10). In the present study, by performing eight extraction steps with the organic phase (0.1M TDdDGA in dodecane), ten steps with an aqueous phase (0.7 M HNO with metals), and six steps with another aqueous phase (0.7 M HNO
with metals), and six steps with another aqueous phase (0.7 M HNO without metals), approximately 99% Dy was recovered into the organic phase with 1% co-extraction of Nd.
without metals), approximately 99% Dy was recovered into the organic phase with 1% co-extraction of Nd.
Sasaki, Yuji; Morita, Keisuke; Kitatsuji, Yoshihiro; Ito, Keisuke*; Yoshizuka, Kazuharu*
Solvent Extraction Research and Development, Japan, 28(2), p.121 - 131, 2021/00
Times Cited Count:2 Percentile:14.88(Chemistry, Multidisciplinary)High concentration of Cs is present in high-level radioactive waste. It is well-known that Cs is an alkali element and difficult to extract completely into an organic phase. Crown ether compounds are widely available for Cs extractants; DtBuDB18C6 (di- -butyl-dibenzo-18crown6), was used in this study. Organic solvents used for the industrial applications, such as
-butyl-dibenzo-18crown6), was used in this study. Organic solvents used for the industrial applications, such as  -dodecane and 1-octanol, have low solubility concerning the compound; other solvents were employed and tested. In this study, ketone-, ether-, and ester-type solvents showed high solubility for DtBuDB18C6 and DtBuDB18C6, when dissolved in ketones and alcohols, exhibited relatively high Cs distribution ratios (
-dodecane and 1-octanol, have low solubility concerning the compound; other solvents were employed and tested. In this study, ketone-, ether-, and ester-type solvents showed high solubility for DtBuDB18C6 and DtBuDB18C6, when dissolved in ketones and alcohols, exhibited relatively high Cs distribution ratios ( (Cs)), closely to 10.
(Cs)), closely to 10.
 system
systemSasaki, Yuji; Morita, Keisuke; Matsumiya, Masahiko*; Nakase, Masahiko*
Radiochimica Acta, 108(9), p.689 - 699, 2020/09
Times Cited Count:9 Percentile:75.92(Chemistry, Inorganic & Nuclear)The simultaneous separation of Am and Cm from lanthanides is important for atomic energy fields. All lanthanides, Am, and Cm can be extracted by diglycolamide (DGA). In addition, relatively high separation factors between An and Ln were obtained by the extraction system of TODGA, DTPA (diethylenetriamine-pentaacetic acid) and HNO . In this work, DTPA-BA (diethylenetriamine-triacetic-bisamide), which is an improved version of DTPA, was employed for the separation of Ln and An. A relatively high separation factor (approximately 8) for actinides/lanthanides was obtained. Then, the multi-step extraction was performed. Thus, the recoveries of 94.7% for Nd and 4.7% for Am and Cm in organic phase, and 5.3% Nd and 95.3% for Am and Cm in aqueous phase were obtained.
. In this work, DTPA-BA (diethylenetriamine-triacetic-bisamide), which is an improved version of DTPA, was employed for the separation of Ln and An. A relatively high separation factor (approximately 8) for actinides/lanthanides was obtained. Then, the multi-step extraction was performed. Thus, the recoveries of 94.7% for Nd and 4.7% for Am and Cm in organic phase, and 5.3% Nd and 95.3% for Am and Cm in aqueous phase were obtained.
Sasaki, Yuji; Ban, Yasutoshi; Morita, Keisuke; Matsumiya, Masahiko*; Ono, Ryoma*; Shiroishi, Hidenobu*
Solvent Extraction Research and Development, Japan, 27(1), p.63 - 67, 2020/00
Times Cited Count:6 Percentile:31.74(Chemistry, Multidisciplinary)Mutual separation technique of Dy and Nd in Nd magnet is studied. Dy is more valuable than Nd, then Dy might be isolated and reused. Lanthanide elements can be extracted thoroughly by diglycolamide (DGA) extractants, we use this reagent for the recovery and isolation of Dy. Tetradodecyl-DGA (TDdDGA) has relatively high separation factors(SF) between Dy and Nd (SF=17-18) in HNO extraction system, counter-current extraction using TDdDGA was applied for their mutual separation. From the present study, using the condition, four extraction stages, organic phase: 0.1M TDdDGA in n-dodecane, aqueous phase: 0.3M HNO
extraction system, counter-current extraction using TDdDGA was applied for their mutual separation. From the present study, using the condition, four extraction stages, organic phase: 0.1M TDdDGA in n-dodecane, aqueous phase: 0.3M HNO , 92% Dy can be recovered with 0.7% co-extraction of Nd.
, 92% Dy can be recovered with 0.7% co-extraction of Nd.
Sasaki, Yuji; Morita, Keisuke; Matsumiya, Masahiko*; Nakase, Masahiko*
Proceedings of International Nuclear Fuel Cycle Conference / Light Water Reactor Fuel Performance Conference (Global/Top Fuel 2019) (USB Flash Drive), p.108 - 112, 2019/09
We attempted to separate An from Ln, and Am and Cm by the system including extractant and masking agent. The separation factor of Nd and Am was approximately 10 by TODGA-DTPA-BA and that of Am and Cm was over 3 by TODGA-DOODA(C2). Using these batch data, profiles of metal concentration with multi-step extractions proposed in this manuscript were demonstrated.
Morita, Keisuke; Suzuki, Hideya; Matsumura, Tatsuro; Takahashi, Yuya*; Omori, Takashi*; Kaneko, Masaaki*; Asano, Kazuhito*
Proceedings of International Nuclear Fuel Cycle Conference / Light Water Reactor Fuel Performance Conference (Global/Top Fuel 2019) (USB Flash Drive), p.464 - 468, 2019/09
High level liquid waste (HLLW) contains several radionuclides with half-lives longer than 10 year. For reduce environmental burden of waste disposal, minor actinoids and long-lived fission products will to be partitioned and transmuted. JAEA and Toshiba developed process for recovering Se, Zr, Pd and Cs from HLLW. Solvent extraction for Zr with novel extractant,
year. For reduce environmental burden of waste disposal, minor actinoids and long-lived fission products will to be partitioned and transmuted. JAEA and Toshiba developed process for recovering Se, Zr, Pd and Cs from HLLW. Solvent extraction for Zr with novel extractant,  -didodecyl-2-hydroxyacetoamide (HAA) was detailed. The HAA system showed high selectivity for Zr, as indicated by the extraction order of Zr
-didodecyl-2-hydroxyacetoamide (HAA) was detailed. The HAA system showed high selectivity for Zr, as indicated by the extraction order of Zr  Mo
Mo  Pd
Pd  Ag
Ag  Sb
Sb  Sn
Sn  Lns
Lns  Fe. The extracted species was determined as Zr(HAA)
Fe. The extracted species was determined as Zr(HAA) (NO
(NO )
) (HNO
(HNO )
) . A continuous countercurrent extraction with HAA was applied to a simulated, concentrated HLLW after Pd, Se, and Cs removal, where the quantitative extraction of Zr and Mo was effectively demonstrated.
. A continuous countercurrent extraction with HAA was applied to a simulated, concentrated HLLW after Pd, Se, and Cs removal, where the quantitative extraction of Zr and Mo was effectively demonstrated.
Sasaki, Yuji; Morita, Keisuke
Progress in Nuclear Science and Technology (Internet), 5, p.27 - 32, 2018/12
no abstracts in English
Sasaki, Yuji; Morita, Keisuke; Saeki, Morihisa*; Hisamatsu, Shugo*; Yoshizuka, Kazuharu*
Proceedings of 21st International Solvent Extraction Conference (ISEC 2017) (Internet), p.131 - 134, 2017/11
Three tridentate extractants including soft and hard donor has been developed and examined. The extractants are termed as 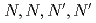 -tetraoctyl-diglycolamide (TODGA), methylimino-
-tetraoctyl-diglycolamide (TODGA), methylimino-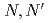 -dioctylacetamide (MIDOA) and
-dioctylacetamide (MIDOA) and 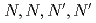 -tetraoctyl-thiodiglycolamide (TDGA). The results of the present study show that TODGA can extract completely lanthanides and actinides, MIDOA can extract palladium, technetium, and rhenium, and TDGA can extract palladium, silver, and gold. We can compare the distribution ratios of these metals by TODGA, MIDOA, and TDGA. These results can be supported by some spectrometric studies, i.e., IR, NMR and UV, and calculations of metal complexes.
-tetraoctyl-thiodiglycolamide (TDGA). The results of the present study show that TODGA can extract completely lanthanides and actinides, MIDOA can extract palladium, technetium, and rhenium, and TDGA can extract palladium, silver, and gold. We can compare the distribution ratios of these metals by TODGA, MIDOA, and TDGA. These results can be supported by some spectrometric studies, i.e., IR, NMR and UV, and calculations of metal complexes.
Sasaki, Yuji; Morita, Keisuke; Ito, Keisuke; Suzuki, Shinichi; Shiwaku, Hideaki; Takahashi, Yuya*; Kaneko, Masaaki*; Omori, Takashi*; Asano, Kazuhito*
Proceedings of International Nuclear Fuel Cycle Conference (GLOBAL 2017) (USB Flash Drive), 4 Pages, 2017/09
no abstracts in English
Sasaki, Yuji; Morita, Keisuke; Suzuki, Shinichi; Shiwaku, Hideaki; Ito, Keisuke; Takahashi, Yuya*; Kaneko, Masaaki*
Solvent Extraction Research and Development, Japan, 24(2), p.113 - 122, 2017/06
The solvent extraction of Se, Zr, Pd, and Cs from nitric acid into 1-octanol (OC) and dodecane has been performed. These elements include long-lived radionuclides in spent nuclear fuels, so a simple separation method is indispensable for the development of the treatment of high-level liquid radioactive waste. It was found that Se can be extracted using phenylenediamine, Zr can be extracted using tetraoctyl diglycolamide and di-2-ethylhexyl phosphoric acid, and Pd can be extracted using (methylimino)bis(dioctylacetamide) and hexaoctylnitrilotriacetamide. These elements can be recovered in over 90% yield by these extractants from nitric acid into OC. A distribution ratio of Cs of greater than 1 can be obtained using di-t-butyldibenzo-18-crown-6. It is clear that 90% recovery of Cs can be achieved using an extraction solvent with ten times the volume of the aqueous phase.
Sasaki, Yuji; Morita, Keisuke; Saeki, Morihisa*; Hisamatsu, Shugo; Yoshizuka, Kazuharu*
Hydrometallurgy, 169, p.576 - 584, 2017/05
Times Cited Count:14 Percentile:56.4(Metallurgy & Metallurgical Engineering)The novel tridentate extractant including soft donor has developed and examined. The extractant, tetraoctyl-thiodiglycolamide (TDGA), is analogous structure to tetraoctyl-diglycolamide (TODGA) and methylimino-dioctylacetamide (MIDOA), but with sulfur donor instead of ether oxygen or nitrogen atoms of TODGA or MIDOA. From the present work, TDGA can extract silver, palladium, gold, and mercury from acidic solutions to n-dodecane. In addition to these results, the distribution ratios of hard and soft acid metals by using TDGA, TODGA, and MIDOA are compared, where the metal-complexations with each donor atom are investigated. 1H-NMR and IR studies for the metal-TDGA complexes indicate the role on donor atoms, S and N, of TDGA.
Suzuki, Tomoya; Morita, Keisuke; Sasaki, Yuji; Matsumura, Tatsuro
Separation Science and Technology, 51(17), p.2815 - 2822, 2016/09
Times Cited Count:7 Percentile:23.23(Chemistry, Multidisciplinary)To understand the adsorption properties of styrene-divinylbenzene copolymer functionalized with 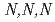 -trimethylglycine, AMP03, the adsorption behaviors for platinoid ions (Ru(III), Rh(III), and Pd(II)) were examined. Furthermore, we performed adsorption experiments using sample solutions with adding triethylamine, thiourea, and
-trimethylglycine, AMP03, the adsorption behaviors for platinoid ions (Ru(III), Rh(III), and Pd(II)) were examined. Furthermore, we performed adsorption experiments using sample solutions with adding triethylamine, thiourea, and 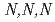 -trimethylglycine. Based on the adsorption data obtained in this study, we performed chromatographic experiments. The results indicated that all platinoid ions in the feed solution completely adsorbed on AMP03, and almost 80% of the adsorbed platinoid ions were recovered. These results show that AMP03 has the potential to recover Ru(III), Rh(III), and Pd(II) from high-level liquid waste.
-trimethylglycine. Based on the adsorption data obtained in this study, we performed chromatographic experiments. The results indicated that all platinoid ions in the feed solution completely adsorbed on AMP03, and almost 80% of the adsorbed platinoid ions were recovered. These results show that AMP03 has the potential to recover Ru(III), Rh(III), and Pd(II) from high-level liquid waste.
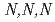 -trimethylglycine
-trimethylglycineSuzuki, Tomoya; Morita, Keisuke; Sasaki, Yuji; Matsumura, Tatsuro
Bulletin of the Chemical Society of Japan, 89(5), p.608 - 616, 2016/05
Times Cited Count:5 Percentile:16.1(Chemistry, Multidisciplinary)In a previous study, we found that AMP03 adsorbs Rh(III) from the HNO solution with pH = 1.7 - 2.2. In this study, to understand the Rh(III) adsorption properties of AMP03 and clarify conditions for efficient recovery, we investigated the adsorption behaviors of Rh(III) from the HNO
solution with pH = 1.7 - 2.2. In this study, to understand the Rh(III) adsorption properties of AMP03 and clarify conditions for efficient recovery, we investigated the adsorption behaviors of Rh(III) from the HNO solution. As a result, it was found that Rh(III) is effectively adsorbed from aqueous solution containing low H
solution. As a result, it was found that Rh(III) is effectively adsorbed from aqueous solution containing low H and high NO
and high NO
 concentration. Considering the adsorption mechanism of Rh(III), we found that Rh
concentration. Considering the adsorption mechanism of Rh(III), we found that Rh in aqueous solution is adsorbed with two betaine groups in AMP03 and three NO
in aqueous solution is adsorbed with two betaine groups in AMP03 and three NO
 .
.
Sasaki, Yuji; Morita, Keisuke; Shimazaki, Shoma*; Tsubata, Yasuhiro; Ozawa, Masaki*
Solvent Extraction Research and Development, Japan, 23(2), p.161 - 174, 2016/05
We examined the masking effects of 16 water-miscible reagents, on the extraction of Mo, Re, Ru, and Pd. The extractants, methylimino-dioctylacetamide (MIDOA) and hexaoctyl-nitrilotriacetamide (NTAamide(C8)), show significantly high distribution ratios for these metals, were employed in this study. Masking effects were observed as a decrease of distribution ratio with an increase of masking agent concentration in these extraction systems. The results showed that Pd and Ru can be masked by similar reagents including N- or S- donor atoms, which suppressed the extraction into the organic phase. In contrast, distribution ratio of Mo was only slightly masked by the above mentioned reagents. The masking of Mo was achieved using complexing agents having a central N(CH C(P)=O)
C(P)=O) framework that is important for this purpose. A masking agent for Re was not found in this study.
framework that is important for this purpose. A masking agent for Re was not found in this study.
 -tetraoctyl-thiodiglycolamide
-tetraoctyl-thiodiglycolamideSasaki, Yuji; Suzuki, Tomoya; Morita, Keisuke; Yoshizuka, Kazuharu*
Hydrometallurgy, 159, p.107 - 109, 2016/01
Times Cited Count:5 Percentile:27.46(Metallurgy & Metallurgical Engineering)The novel tridentate extractant including soft donor has developed and examined. The extractant, tetraoctyl-thiodiglycolamide (S-DGA), is analogous structure to tetraoctyl-diglycolamide (TODGA), but with sulfur donor instead of ether oxygen of TODGA. S-DGA has unique property to extract silver from acidic solutions to n-dodecane with relatively high D values. We investigated the extraction behavior of Ag in various acids, HNO , H
, H SO
SO , and HClO
, and HClO .
.
Sasaki, Yuji; Sugo, Yumi; Morita, Keisuke; Nash, K.*; Nash, K. L.*
Solvent Extraction and Ion Exchange, 33(7), p.625 - 641, 2015/10
Times Cited Count:95 Percentile:91.19(Chemistry, Multidisciplinary)The effect of alkyl substituents at amidic N atoms in diglycolamide (DGA) compounds on solvent extraction was investigated. The solubility in water and n-dodecane, loading capacity, and distribution ratio (D) of lanthanides and actinides for various DGA compounds were monitored. DGA derivatives with short alkyl chains, for example methyl and ethyl groups, are highly water soluble, while DGA derivatives with long alkyl chains are highly soluble in n-dodecane. The loading capacity correlats with their alkyl chain lengths. The results of masking effect and solubility tests indicate that TEDGA(tetraethyl-diglycolamide) is the best actinide masking agent among the water-soluble DGA derivatives. Actinide extractions were performed using ten DGA compounds in six diluents (nitrobenzene, 1,2-dichloroethane, 1-octanol, chloroform, toluene, and n-dodecane), in which it was observed that DGA derivatives with shorter alkyl chains show higher D values.
Sasaki, Yuji; Tsubata, Yasuhiro; Shirasu, Noriko; Morita, Keisuke; Suzuki, Tomoya
Nihon Genshiryoku Gakkai Wabun Rombunshi, 14(3), p.202 - 212, 2015/09
We have been developing the new partitioning method of high-level radioactive waste by single-cycle extraction process. This process is composed of extraction of actinides (An) and fission products (FP, e.g., Pd, Ru, Mo and Tc), and mutual separation by back-extraction. The extractant employed in this process is required to extract soft, hard acid metals and oxonium anions simultaneously. The NTAamide (hexaoctyl-nitrilotriacetamide) is one of the candidate extractants. After extraction of An and FP, the mutual separation by back-extraction should be set up. Pd and Ru extracted by NTAamide can be back-extracted by complexing agents such as thiourea, systeine, diethylenetriamine, and trisaminoethylamine, and the back-extraction of Mo can be performed by methylimino-diethylacetamide (MIDEA), NTAamide(C2) and iminodimethylphosphoric acid, and Re can be done by aqueous phase with high pH.
Sasaki, Yuji; Tsubata, Yasuhiro; Shirasu, Noriko; Morita, Keisuke; Suzuki, Tomoya
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.1653 - 1656, 2015/09
The concept for the new partitioning method of high-level radioactive waste (HLW) by single-cycle extraction process has been investigated. This process is based on extraction of actinides (An) and fission products (FP), and mutual separation by reverse extraction. Solo extractant and several stripping reagents will be utilized in this process. The extractant employed in this process is required to extract soft (platinum metals), hard acid metals(An), and oxonium anions (Mo, Tc) simultaneously. NTAamide is one of the candidate extractants. After extraction of An and FP by NTAamide(C8), the mutual separation among these metals by reverse extraction will be followed using the suitable water-miscible reagents. The extraction of An and FP, and the masking effect by some water-miscible reagents has been studied.
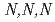 -trimethylglycine to Ru(III), Rh(III) and Pd(II) for developing separation techniques from high-level liquid waste
-trimethylglycine to Ru(III), Rh(III) and Pd(II) for developing separation techniques from high-level liquid wasteSuzuki, Tomoya; Shimazaki, Shoma*; Morita, Keisuke; Sasaki, Yuji; Ozawa, Masaki*; Matsumura, Tatsuro
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.1539 - 1543, 2015/09
To understand the adsorption behaviors of ion-exchange resin bearing 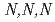 -trimethylglycine groups (AMP03), adsorption experiments using HNO
-trimethylglycine groups (AMP03), adsorption experiments using HNO solutions containing Ru(III), Rh(III), and Pd(II) have been performed. AMP03 strongly adsorbed Pd(II), and Ru(III) and Rh(III) were moderately adsorbed. To control the adsorption abilities of AMP03 for the Ru(III), Rh(III) and Pd(II), adsorption experiments using sample solutions with added thiourea (TU), triethylamine (TEA), and betaine anhydrous were performed. The adsorption abilities for Ru(III) and Rh(III) greatly increased with the addition of TEA, whereas the ability to adsorb Pd(II) was not significantly affected. The addition of TU or betaine anhydrous remarkably decreased the adsorption ability for Pd(II), and contrastingly slight changes in the abilities to adsorb Ru and Rh were also observed. These results show that AMP03 has a significant potential for separation of platinoid elements from HNO
solutions containing Ru(III), Rh(III), and Pd(II) have been performed. AMP03 strongly adsorbed Pd(II), and Ru(III) and Rh(III) were moderately adsorbed. To control the adsorption abilities of AMP03 for the Ru(III), Rh(III) and Pd(II), adsorption experiments using sample solutions with added thiourea (TU), triethylamine (TEA), and betaine anhydrous were performed. The adsorption abilities for Ru(III) and Rh(III) greatly increased with the addition of TEA, whereas the ability to adsorb Pd(II) was not significantly affected. The addition of TU or betaine anhydrous remarkably decreased the adsorption ability for Pd(II), and contrastingly slight changes in the abilities to adsorb Ru and Rh were also observed. These results show that AMP03 has a significant potential for separation of platinoid elements from HNO solution.
solution.