Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nagai, Yuki; Shinaoka, Hiroshi*
Journal of the Physical Society of Japan, 92(3), p.034703_1 - 034703_8, 2023/03
Times Cited Count:0 Percentile:0(Physics, Multidisciplinary)no abstracts in English
Wallerberger, M.*; Badr, S.*; Hoshino, Shintaro*; Huber, S.*; Kakizawa, Fumiya*; Koretsune, Takashi*; Nagai, Yuki; Nogaki, Kosuke*; Nomoto, Takuya*; Mori, Hitoshi*; et al.
Software X (Internet), 21, p.101266_1 - 101266_7, 2023/02
Times Cited Count:10 Percentile:87.16(Computer Science, Software Engineering)no abstracts in English
Shinaoka, Hiroshi*; Nagai, Yuki
Physical Review B, 103(4), p.045120_1 - 045120_8, 2021/01
Times Cited Count:3 Percentile:26.56(Materials Science, Multidisciplinary)no abstracts in English
Nagai, Takayuki; Okamoto, Yoshihiro; Akiyama, Daisuke*; Uehara, Akihiro*; Fujii, Toshiyuki*; Sekimoto, Shun*
KURNS Progress Report 2019, P. 257, 2020/08
To understand the influence of glass structural change by neutron irradiation and boron isotope composition, glass samples were made from enrichment boric acid reagents and neutron irradiation of those samples was carried out in Pn-2 of KUR. The structural change of glass sample after the irradiation will be estimated in 2020FY. Before neutron irradiation test of glass samples, the Si-O bridging structure difference by boron isotope composition compared by using a Raman spectrometry.
Nagai, Takayuki; Kobayashi, Hidekazu; Okamoto, Yoshihiro; Akiyama, Daisuke*; Sato, Nobuaki*; Uehara, Akihiro*; Fujii, Toshiyuki*; Sekimoto, Shun*
KURNS Progress Report 2018, P. 105, 2019/08
To understand this structural change of a borosilicate glass by a neutron irradiation in detail, the irradiation test was carried out in KUR in 2017FY. The glass structure was estimated by using Raman spectrometry in 2018FY. Comparing with the Raman spectra of glass samples before and after irradiation, it could be observed the change of peak height of Si-O bridging structure by the irradiation.
Nagai, Yuki; Shinaoka, Hiroshi*
Journal of the Physical Society of Japan, 88(6), p.064004_1 - 064004_5, 2019/06
Times Cited Count:11 Percentile:64.45(Physics, Multidisciplinary)no abstracts in English
Nagai, Takayuki; Uehara, Akihiro*; Fujii, Toshiyuki*
KURRI Progress Report 2017, P. 128, 2018/08
In this study, to understand the structural change of a borosilicate glass by a neutron irradiation in detail, the neutron irradiation test was carried out for 50 min in the Pn-2 of the Kyoto University Research Reactor (KUR). The structural change of glass sample after the irradiation will be estimated in 2018FY. Before the irradiation test, the glass composition was selected to estimate a structural change accurately by Raman spectrometry and 2 kinds of Li free borosilicate glass for the irradiation test samples were prepared.
 VAl probed by soft X-ray spectroscopies
VAl probed by soft X-ray spectroscopiesNagai, Kodai*; Fujiwara, Hidenori*; Aratani, Hidekazu*; Fujioka, Shuhei*; Yomosa, Hiroshi*; Nakatani, Yasuhiro*; Kiss, Takayuki*; Sekiyama, Akira*; Kuroda, Fumiaki*; Fujii, Hitoshi*; et al.
Physical Review B, 97(3), p.035143_1 - 035143_8, 2018/01
Times Cited Count:22 Percentile:69.75(Materials Science, Multidisciplinary)We have studied the electronic structure of ferrimagnetic Mn VAl single crystals by means of soft X-ray absorption spectroscopy (XAS), X-ray absorption magnetic circular dichroism (XMCD), and resonant soft X-ray inelastic scattering (RIXS). We have successfully observed the XMCD signals for all the constituent elements. The Mn L
VAl single crystals by means of soft X-ray absorption spectroscopy (XAS), X-ray absorption magnetic circular dichroism (XMCD), and resonant soft X-ray inelastic scattering (RIXS). We have successfully observed the XMCD signals for all the constituent elements. The Mn L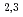 XAS and XMCD spectra are reproduced by spectral simulations based on density-functional theory, indicating the itinerant character of the Mn 3
XAS and XMCD spectra are reproduced by spectral simulations based on density-functional theory, indicating the itinerant character of the Mn 3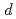 states. On the other hand, the V 3
states. On the other hand, the V 3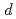 electrons are rather localized since the ionic model can qualitatively explain the V L
electrons are rather localized since the ionic model can qualitatively explain the V L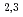 XAS and XMCD spectra. This picture is consistent with local
XAS and XMCD spectra. This picture is consistent with local 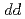 excitations revealed by the V L
excitations revealed by the V L RIXS.
RIXS.
Nagai, Takayuki; Kobayashi, Hidekazu; Okamoto, Yoshihiro; Uehara, Akihiro*; Fujii, Toshiyuki*
Photon Factory Activity Report 2015, Part B, 2 Pages, 2016/00
To investigate the characterization damage of a borosilicate glass by a neutron irradiation, the glass sample after neutron irradiation was estimated by using the Raman spectrophotometry and the synchrotron XAFS measurement. As a result, we confirmed that the Si-O bridge structure of a borosilicate glass and the containing element valence in the glass were changed by the neutron irradiation.
Hattori, Takanori; Sano, Asami; Arima, Hiroshi*; Komatsu, Kazuki*; Yamada, Akihiro*; Inamura, Yasuhiro; Nakatani, Takeshi; Seto, Yusuke*; Nagai, Takaya*; Utsumi, Wataru; et al.
Nuclear Instruments and Methods in Physics Research A, 780, p.55 - 67, 2015/04
Times Cited Count:75 Percentile:98.98(Instruments & Instrumentation)PLANET is a time-of-flight (ToF) neutron beamline dedicated to high-pressure and high-temperature experiments. The large six-axis multi-anvil high-pressure press designed for ToF neutron diffraction experiments enables routine data collection at high pressures and high temperatures up to 10 GPa and 2000 K, respectively. To obtain clean data, the beamline is equipped with the incident slits and receiving collimators to eliminate parasitic scattering from the high-pressure cell assembly. The high performance of the diffractometer for the resolution (
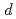 /
/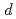
 0.6%) and the accessible
0.6%) and the accessible 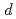 -spacing range (0.2-8.4
-spacing range (0.2-8.4  ) together with low-parasitic scattering characteristics enables precise structure determination of crystals and liquids under high pressure and temperature conditions.
) together with low-parasitic scattering characteristics enables precise structure determination of crystals and liquids under high pressure and temperature conditions.
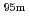 Tc for Compton camera imaging
Tc for Compton camera imagingHatsukawa, Yuichi; Hashimoto, Kazuyuki; Tsukada, Kazuaki; Sato, Tetsuya; Asai, Masato; Toyoshima, Atsushi; Nagai, Yasuki; Tanimori, Toru*; Sonoda, Shinya*; Kabuki, Shigeto*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1283 - 1285, 2015/02
Times Cited Count:2 Percentile:17.52(Chemistry, Analytical)Technetium-99m ( Tc) is used in radioactive medical diagonostic tests, for example as a radioactive tracer that medical equipment can detect in the human body. It is well suited to the role because it emits readily detectable 141 keV
Tc) is used in radioactive medical diagonostic tests, for example as a radioactive tracer that medical equipment can detect in the human body. It is well suited to the role because it emits readily detectable 141 keV  rays, and its half-life is 6.01 hours (meaning that about 94% of it decays to technetium-99 in 24 hours). There are at least 31 commonly used radiopharmaceuticals based on technetium-99m for imaging and functional studies of the brain, myocardium, thyroid, lungs, liver, gallbladder, kidneys, skeleton, blood, and tumors. Recent years, with the develop-ment of the Compton camera which can realize high position resolution, technetium isotopes emitting high energy
rays, and its half-life is 6.01 hours (meaning that about 94% of it decays to technetium-99 in 24 hours). There are at least 31 commonly used radiopharmaceuticals based on technetium-99m for imaging and functional studies of the brain, myocardium, thyroid, lungs, liver, gallbladder, kidneys, skeleton, blood, and tumors. Recent years, with the develop-ment of the Compton camera which can realize high position resolution, technetium isotopes emitting high energy  -rays are required. In this study, technetium-95m which emits some
-rays are required. In this study, technetium-95m which emits some  rays around 800 keV was produced by the
rays around 800 keV was produced by the 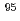 Mo(p,n)
Mo(p,n)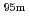 Tc reaction.
Tc reaction.
 /UO
/UO couple in Li
couple in Li MoO
MoO -Na
-Na MoO
MoO eutectic melt at 550
eutectic melt at 550 C
CNagai, Takayuki; Uehara, Akihiro*; Fujii, Toshiyuki*; Sato, Nobuaki*; Kofuji, Hirohide; Myochin, Munetaka; Yamana, Hajimu*
Journal of Nuclear Materials, 454(1-3), p.159 - 163, 2014/11
Times Cited Count:1 Percentile:8.82(Materials Science, Multidisciplinary)The redox equilibrium of UO /UO
/UO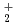 couple was measured in Li
couple was measured in Li MoO
MoO -Na
-Na MoO
MoO eutectic melt at 550
eutectic melt at 550 C by cyclic voltammetry and absorption spectrophotometry. The standard redox potential of UO
C by cyclic voltammetry and absorption spectrophotometry. The standard redox potential of UO /UO
/UO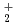 couple was approximately evaluated by cyclic voltammetry. Further, the absorption spectrum and equilibrium potential were measured, repeatedly adding UO
couple was approximately evaluated by cyclic voltammetry. Further, the absorption spectrum and equilibrium potential were measured, repeatedly adding UO source material into the melt containing UO
source material into the melt containing UO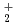 . From the correlation between the equilibrium potential of the melt and the concentration ratio [UO
. From the correlation between the equilibrium potential of the melt and the concentration ratio [UO ]/[UO
]/[UO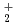 ] spectrophotometrically evaluated, the standard redox potential of UO
] spectrophotometrically evaluated, the standard redox potential of UO /UO
/UO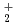 couple was determined to be -0.847
couple was determined to be -0.847 0.010 V vs. O
0.010 V vs. O /O
/O .
.

 in molten LiCl-RbCl and LiCl-KCl eutectics by using tungsten
in molten LiCl-RbCl and LiCl-KCl eutectics by using tungstenNagai, Takayuki; Uehara, Akihiro*; Fujii, Toshiyuki*; Yamana, Hajimu*
Journal of Nuclear Materials, 439(1-3), p.1 - 6, 2013/08
Times Cited Count:9 Percentile:53.27(Materials Science, Multidisciplinary)The reduction of uranium from UO
 to UO
to UO
 or U
or U in molten LiCl-RbCl and LiCl-KCl eutectics was examined by using tungsten and chlorine gas. Spectrophotometric technique was adopted to determine the concentration of uranium species. When tungsten was immersed into the LiCl-RbCl eutectic melt at 400
in molten LiCl-RbCl and LiCl-KCl eutectics was examined by using tungsten and chlorine gas. Spectrophotometric technique was adopted to determine the concentration of uranium species. When tungsten was immersed into the LiCl-RbCl eutectic melt at 400 C without supplying chlorine gas, 36% of the total weight of the hexavalent of UO
C without supplying chlorine gas, 36% of the total weight of the hexavalent of UO
 was reduced to the pentavalent of UO
was reduced to the pentavalent of UO
 . Under purging chlorine gas into the melt, 96% of UO
. Under purging chlorine gas into the melt, 96% of UO
 was reduced to the tetravalent of U
was reduced to the tetravalent of U . Tungsten oxy-chloride of WOCl
. Tungsten oxy-chloride of WOCl was produced via the reductions of UO
was produced via the reductions of UO
 , which was volatized from the melt and adsorbed on the upper part of experimental cell. On the other hand, 84% of UO
, which was volatized from the melt and adsorbed on the upper part of experimental cell. On the other hand, 84% of UO
 in the LiCl-KCl eutectic melt at 500
in the LiCl-KCl eutectic melt at 500 C was reduced to U
C was reduced to U by using tungsten and chlorine gas.
by using tungsten and chlorine gas.
 MoO
MoO
Nagai, Takayuki; Sato, Nobuaki*; Kitawaki, Shinichi; Uehara, Akihiro*; Fujii, Toshiyuki*; Yamana, Hajimu*; Myochin, Munetaka
Journal of Nuclear Materials, 433(1-3), p.397 - 403, 2013/02
Times Cited Count:10 Percentile:60.97(Materials Science, Multidisciplinary)In order to examine easily synthetic conditions of uranyl molybdate, UO MoO
MoO , used for the reprocessing process study of spent nuclear oxide fuels in alkaline molybdate melts, the uranium molybdate compounds were produced from U
, used for the reprocessing process study of spent nuclear oxide fuels in alkaline molybdate melts, the uranium molybdate compounds were produced from U O
O powder and anhydrous MoO
powder and anhydrous MoO reagent. The results of having investigated them in solid state by using X-ray diffractometry and Raman spectrometry, it was confirmed that UO
reagent. The results of having investigated them in solid state by using X-ray diffractometry and Raman spectrometry, it was confirmed that UO MoO
MoO could be synthesized by heating mixed powder of U
could be synthesized by heating mixed powder of U O
O and MoO
and MoO with stoichiometric mole ratio at 770
with stoichiometric mole ratio at 770 C for 4 h under air atmosphere. Moreover, adding this UO
C for 4 h under air atmosphere. Moreover, adding this UO MoO
MoO into Li
into Li MoO
MoO -Na
-Na MoO
MoO eutectic melt, most of the dissolved uranium species in the melt were observed as hexa-valent uranyl ions by absorption spectrophotometry.
eutectic melt, most of the dissolved uranium species in the melt were observed as hexa-valent uranyl ions by absorption spectrophotometry.
 C
CNagai, Takayuki; Uehara, Akihiro*; Fukushima, Mineo; Myochin, Munetaka; Fujii, Toshiyuki*; Sato, Nobuaki*; Yamana, Hajimu*
Proceedings in Radiochemistry, 1(1), p.151 - 155, 2011/09
Absorption spectra of dissolved uranium species in molten Li MoO
MoO -Na
-Na MoO
MoO eutectic at 550
eutectic at 550  C were measured by spectrophotometry, and their redox reactions were also investigated by cyclic voltammetry. Observed absorption spectra of uranium species were similar to those of UO
C were measured by spectrophotometry, and their redox reactions were also investigated by cyclic voltammetry. Observed absorption spectra of uranium species were similar to those of UO
 in molten chlorides. After purging oxygen into the melt, the absorption peaks of UO
in molten chlorides. After purging oxygen into the melt, the absorption peaks of UO
 decreased and UO
decreased and UO
 was thought to be oxidized to UO
was thought to be oxidized to UO
 . When the uranium species were not contained in the melt, we confirmed that alkali metals deposited at -0.7 V and a small reduction of this melt was observed at -0.3 V. When UO
. When the uranium species were not contained in the melt, we confirmed that alkali metals deposited at -0.7 V and a small reduction of this melt was observed at -0.3 V. When UO was dissolved into the melt, the reduction of the uranium species was observed at -0.2 V. It was suggested that the dissolved uranium species are recovered as mixed uranium-molybdenum oxides by electrolysis.
was dissolved into the melt, the reduction of the uranium species was observed at -0.2 V. It was suggested that the dissolved uranium species are recovered as mixed uranium-molybdenum oxides by electrolysis.
 solutions
solutionsUehara, Akihiro*; Fujii, Toshiyuki*; Matsuura, Haruaki*; Sato, Nobuaki*; Nagai, Takayuki; Minato, Kazuo; Yamana, Hajimu*; Okamoto, Yoshihiro
Proceedings in Radiochemistry, 1(1), p.161 - 165, 2011/09
Nagai, Takayuki; Uehara, Akihiro*; Fujii, Toshiyuki*; Sato, Nobuaki*; Yamana, Hajimu*
Journal of Nuclear Materials, 414(2), p.226 - 231, 2011/07
Times Cited Count:11 Percentile:63.92(Materials Science, Multidisciplinary)Sakanaka, Shogo*; Akemoto, Mitsuo*; Aoto, Tomohiro*; Arakawa, Dai*; Asaoka, Seiji*; Enomoto, Atsushi*; Fukuda, Shigeki*; Furukawa, Kazuro*; Furuya, Takaaki*; Haga, Kaiichi*; et al.
Proceedings of 1st International Particle Accelerator Conference (IPAC '10) (Internet), p.2338 - 2340, 2010/05
Future synchrotron light source using a 5-GeV energy recovery linac (ERL) is under proposal by our Japanese collaboration team, and we are conducting R&D efforts for that. We are developing high-brightness DC photocathode guns, two types of cryomodules for both injector and main superconducting (SC) linacs, and 1.3 GHz high CW-power RF sources. We are also constructing the Compact ERL (cERL) for demonstrating the recirculation of low-emittance, high-current beams using above-mentioned critical technologies.
 /U
/U and U
and U /U couples in molten LiCl-RbCl eutectic
/U couples in molten LiCl-RbCl eutecticNagai, Takayuki; Uehara, Akihiro*; Fujii, Toshiyuki*; Shirai, Osamu*; Sato, Nobuaki*; Yamana, Hajimu*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 77(8), p.614 - 616, 2009/08
Times Cited Count:15 Percentile:32.97(Electrochemistry)Formal redox potentials of the U /U
/U and U
and U /U couples in molten LiCl-RbCl eutectic were determined by cyclic voltammetry. These redox potentials were more negative than those in the LiCl-KCl eutectic but positive comparing to those in the NaCl-CsCl eutectic. This relation would be correlated with the averaged alkali cation radius.
/U couples in molten LiCl-RbCl eutectic were determined by cyclic voltammetry. These redox potentials were more negative than those in the LiCl-KCl eutectic but positive comparing to those in the NaCl-CsCl eutectic. This relation would be correlated with the averaged alkali cation radius.
 /Pu
/Pu and PuO
and PuO
 /Pu
/Pu couples in molten NaCl-CsCl eutectic as measured by absorption spectrophotometry
couples in molten NaCl-CsCl eutectic as measured by absorption spectrophotometryNagai, Takayuki; Uehara, Akihiro*; Fujii, Toshiyuki*; Shirai, Osamu*; Myochin, Munetaka; Yamana, Hajimu*
Radiochimica Acta, 97(4-5), p.209 - 212, 2009/05
Times Cited Count:3 Percentile:24.47(Chemistry, Inorganic & Nuclear)Redox behavior of uranium and plutonium in molten salts is the essential information for establishing an effective reprocessing process with spent MOX fuel. In an oxide electro-winning reprocessing process, the spent nuclear fuel is dissolved into the molten NaCl-CsCl eutectic, and the dissolved U(VI) and Pu(VI) species are reduced to be dioxides by electrolysis. In the present study, formal redox potentials of the Pu /Pu
/Pu and PuO
and PuO
 /Pu
/Pu couples in the molten NaCl-CsCl eutectic at 923 K were determined. The fractions of Pu
couples in the molten NaCl-CsCl eutectic at 923 K were determined. The fractions of Pu , Pu
, Pu , and PuO
, and PuO
 were determined by UV/Vis/NIR absorption spectrophotometry. PuO
were determined by UV/Vis/NIR absorption spectrophotometry. PuO was dissolved into the molten NaCl-CsCl eutectic by Cl
was dissolved into the molten NaCl-CsCl eutectic by Cl
 gas purging. Valences of plutonium (Pu
gas purging. Valences of plutonium (Pu , Pu
, Pu , Pu
, Pu , and PuO
, and PuO
 ) were controlled by changing the flow rates of Cl
) were controlled by changing the flow rates of Cl , O
, O , and Ar gases.
, and Ar gases.