Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Fujii, Toshiyuki*; Egusa, Soichiro*; Uehara, Akihiro*; Yamana, Hajimu*; Morita, Yasuji
Journal of Radioanalytical and Nuclear Chemistry, 295(3), p.2059 - 2062, 2013/03
Times Cited Count:3 Percentile:18.63(Chemistry, Analytical)Quantitative analysis of Nd, U, or Pd in 3 mol dm (M) HNO
(M) HNO was performed by reflection absorption spectrophotometry at ultraviolet-visible-near-infrared (UV/Vis/NIR) region. A sample chamber with optics for reflection measurement was designed and attached to a UV/Vis/NIR spectrophotometer by optical fibers. The reflection absorbance showed linear relations with concentrations of Nd, U, and Pd at the absorbance region less than 0.1. The quantitative analysis was found to be possible for 3 M HNO
was performed by reflection absorption spectrophotometry at ultraviolet-visible-near-infrared (UV/Vis/NIR) region. A sample chamber with optics for reflection measurement was designed and attached to a UV/Vis/NIR spectrophotometer by optical fibers. The reflection absorbance showed linear relations with concentrations of Nd, U, and Pd at the absorbance region less than 0.1. The quantitative analysis was found to be possible for 3 M HNO solutions containing [Nd] ca. 0.2 M, [U] ca. 0.04 M, or [Pd] ca. 0.01 M at maximum.
solutions containing [Nd] ca. 0.2 M, [U] ca. 0.04 M, or [Pd] ca. 0.01 M at maximum.
Fujii, Toshiyuki*; Egusa, Soichiro*; Uehara, Akihiro*; Kirishima, Akira*; Yamagishi, Isao; Morita, Yasuji; Yamana, Hajimu*
Journal of Radioanalytical and Nuclear Chemistry, 290(2), p.475 - 478, 2011/11
Times Cited Count:19 Percentile:80.18(Chemistry, Analytical)Palladium complexation in concentrated nitric acid solutions was studied by UV/Vis absorption spectrophotometry. The ionic strength of the solutions was fixed to 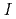 = 1, 3, or 5 M by mixing of HNO
= 1, 3, or 5 M by mixing of HNO and HClO
and HClO . The major palladium species were found to be Pd
. The major palladium species were found to be Pd , PdNO
, PdNO
 , and Pd(NO
, and Pd(NO )
) . The formation constant of PdNO
. The formation constant of PdNO
 was determined to be
was determined to be 
 = 1.32 (
= 1.32 (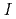 = 1 M), 1.49 (
= 1 M), 1.49 (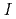 = 3 M), or 1.47 (
= 3 M), or 1.47 (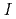 = 5 M), while that of Pd(NO
= 5 M), while that of Pd(NO )
) to be
to be 
 = 0.45 (
= 0.45 (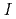 = 3 M) or 0.14 (
= 3 M) or 0.14 (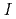 = 5 M).
= 5 M).
Egusa, Soichiro*; Fujii, Toshiyuki*; Uehara, Akihiro*; Yamana, Hajimu*; Yamagishi, Isao; Morita, Yasuji
Kyoto Daigaku Genshiro Jikkenjo Dai-45-Kai Gakujutsu Koenkai Hobunshu, p.123 - 125, 2011/01
Speciation of palladium in nitric acid solutions was studied through spectrophotometric methods to obtain basic data for development of Pd separation process. Stability constants of nitrate complexes of Pd were determined by analysis of absorption spectra of nitric acid solutions of various concentrations with the constant ionic strength.
Egusa, Soichiro*; Fujii, Toshiyuki*; Uehara, Akihiro*; Yamana, Hajimu*; Morita, Yasuji
no journal, ,
In order to investigate chemical species of Pd in nitric acid solution, absorption spectra of Pd were measured by changing nitric acid concentration, origin of Pd and time after preparing the solution. Absorption peak at 430nm increased with the nitric acid solution. Time dependence of the absorption spectra indicated very slow reaction between Pd and nitric acid ion.
Egusa, Soichiro*; Uehara, Akihiro*; Fujii, Toshiyuki*; Yamana, Hajimu*; Yamagishi, Isao; Morita, Yasuji
no journal, ,
Speciation of palladium in nitric acid solutions was studied through spectrophotometric and electrochemical methods to obtain basic data for development of Pd separation process.
Egusa, Soichiro*; Fujii, Toshiyuki*; Uehara, Akihiro*; Yamana, Hajimu*; Yamagishi, Isao; Morita, Yasuji
no journal, ,
Speciation of palladium, molybdenum and ruthenium in nitric acid solutions was studied through spectrophotometric method to obtain basic data for the development of separation process for these elements from high-level liquid waste. Absorption spectra of various solutions of Pd and Mo gave data on complex formation constants and molar absorption coefficients for some chemical species. As for nitric acid solution of Ru, slow rate of change in absorption spectra was observed, which depends on the nitric acid concentration.