Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Inagaki, Yaohiro*; Makigaki, Hikaru; Idemitsu, Kazuya*; Arima, Tatsumi*; Mitsui, Seiichiro; Noshita, Kenji*
Journal of Nuclear Science and Technology, 49(4), p.438 - 449, 2012/04
Times Cited Count:19 Percentile:79.52(Nuclear Science & Technology)Dissolution tests were performed for a simulated HLW glass by using a Micro-Channel Flow-Through (MCFT) test to evaluate the initial dissolution rate, 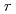
 , as a function of pH and temperature. The results indicated that the
, as a function of pH and temperature. The results indicated that the 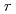
 shows a "V-shaped" pH dependence at 25
shows a "V-shaped" pH dependence at 25 C, which is almost consistent with the previous results measured by using other test methods including Single Pass Flow-Through (SPFT) test. At elevated temperatures, however, the
C, which is almost consistent with the previous results measured by using other test methods including Single Pass Flow-Through (SPFT) test. At elevated temperatures, however, the 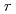
 shows a "U-shaped" pH dependence with a flat bottom at neutral pH, which differs from the previous results. The results also indicated that the MCFT provided a higher value of the
shows a "U-shaped" pH dependence with a flat bottom at neutral pH, which differs from the previous results. The results also indicated that the MCFT provided a higher value of the 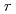
 with a steep slope of pH dependence than the SPFT results at basic pH from 8 to 11 at 90
with a steep slope of pH dependence than the SPFT results at basic pH from 8 to 11 at 90 C. With respect to the temperature dependence, the
C. With respect to the temperature dependence, the 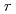
 increases with temperature according to an Arrhenius law at any pH, and the apparent activation energy increases with pH, which suggests that the dissolution mechanism can change depending on pH.
increases with temperature according to an Arrhenius law at any pH, and the apparent activation energy increases with pH, which suggests that the dissolution mechanism can change depending on pH.
Inagaki, Yaohiro*; Mitsui, Seiichiro; Makigaki, Hikaru*; Idemitsu, Kazuya*; Arima, Tatsumi*; Bamba, Tsunetaka; Noshita, Kenji*
Materials Research Society Symposium Proceedings, Vol.1193, p.219 - 228, 2009/05
We developed a new flow-through test method using micro-reactor, and applied it to measurement of the dissolution/alteration kinetics for a simulated HLW glass (P0798). In this method, a glass coupon is placed just on a Teflon plate having a micro-channel, and a solution is injected into the inlet of micro-channel by micro-syringe pump at a constant flow rate. The injected solution flows through the micro-channel reacting with the glass to the outlet, and the outlet solution is retrieved at certain intervals to be analyzed for determination of the dissolution/alteration rate. This method has some major features, i.e., simple test apparatus with compact size, high S/V ratio, sensitive/precise measurement of the glass dissolution/alteration rate, adequate glass shape for analysis of reacted glass surface, and so on. By use of this method the dissolution/alteration rate for P0798 was measured as a function of pH, temperature, flow rate, and time, and some available results were obtained to evaluate the dissolution/alteration kinetics.
Makigaki, Hikaru*; Inagaki, Yaohiro*; Idemitsu, Kazuya*; Arima, Tatsumi*; Mitsui, Seiichiro; Bamba, Tsunetaka; Noshita, Kenji*
Materials Research Society Symposium Proceedings, Vol.1193, p.307 - 314, 2009/05
By using micro-reactor flow-through test method we measured the initial dissolution rate of P0798 glass at 25 C as a function of pH between 5.6 and 12. The results showed that the initial dissolution rate determined by dissolution rate of Si has "V-shaped" pH dependency similar to R7T7 glass reported by CEA, France. We also measured the initial dissolution rate at pH 5.6 as a function of temperature between 25 and 90
C as a function of pH between 5.6 and 12. The results showed that the initial dissolution rate determined by dissolution rate of Si has "V-shaped" pH dependency similar to R7T7 glass reported by CEA, France. We also measured the initial dissolution rate at pH 5.6 as a function of temperature between 25 and 90 C, and the activation energy was evaluated to be 51 kJ/mol, which value is slightly smaller than that of R7T7 glass at pH 9 reported by CEA. On the basis of these results and comparison, we discussed the dissolution kinetics of P0798 glass.
C, and the activation energy was evaluated to be 51 kJ/mol, which value is slightly smaller than that of R7T7 glass at pH 9 reported by CEA. On the basis of these results and comparison, we discussed the dissolution kinetics of P0798 glass.
Inagaki, Yaohiro*; Makigaki, Hikaru*; Idemitsu, Kazuya*; Arima, Tatsumi*; Mitsui, Seiichiro; Bamba, Tsunetaka; Noshita, Kenji*
no journal, ,
Dissolution/alteration of HLW glass and corresponding radionuclide release will be greatly affected by environmental conditions of repository. For reliable performance assessment, it is necessary to evaluate glass dissolution/alteration behavior at various environmental conditions systematically and kinetically. For this purpose, we conducted glass dissolution/alteration tests on simulated waste glass(P0798) at several conditions by use of a newly-developed micro-reactor flow-through apparatus, and evaluated the pH dependence of the initial dissolution rates.
Inagaki, Yaohiro*; Makigaki, Hikaru*; Mitsui, Seiichiro; Idemitsu, Kazuya*; Arima, Tatsumi*; Noshita, Kenji*
no journal, ,
A new type of flow-through test method using micro-reactor was developed and applied to measurement of dissolution kinetics for a Japanese simulated HLW glass, P0798. In this method, a coupon shaped glass is placed on a Teflon plate with a microchannel (20 mm  2 mm
2 mm  0.16 mm), and a solution is injected into the inlet of the channel at a constant flow rate. The outlet solution, which reacted with glass through the channel, is retrieved to be analyzed for determination of the dissolution rate. By using this method we measured the initial dissolution rate as a function of pH and temperature. The results showed that the initial dissolution rate has "V-shaped" pH dependence at any temperature from 25 to 90
0.16 mm), and a solution is injected into the inlet of the channel at a constant flow rate. The outlet solution, which reacted with glass through the channel, is retrieved to be analyzed for determination of the dissolution rate. By using this method we measured the initial dissolution rate as a function of pH and temperature. The results showed that the initial dissolution rate has "V-shaped" pH dependence at any temperature from 25 to 90  C similar to that for R7T7 glass evaluated by CEA, France. However, a certain difference was observed between them in the temperature dependence at high pHs. Based on the results and comparison, we discussed the dissolution kinetics.
C similar to that for R7T7 glass evaluated by CEA, France. However, a certain difference was observed between them in the temperature dependence at high pHs. Based on the results and comparison, we discussed the dissolution kinetics.
Sakatani, Keiichi*; Inagaki, Yaohiro*; Makigaki, Hikaru; Idemitsu, Kazuya*; Arima, Tatsumi*; Mitsui, Seiichiro; Noshita, Kenji*
no journal, ,
A new type of flow-through test method using micro-reactor was developed and applied to measurement of dissolution kinetics for a Japanese simulated HLW glass, P0798. By using this method we measured the initial dissolution rate as a function of pH and temperature. The results showed that the initial dissolution rate has "V-shaped" pH dependence at any temperature and Arrhenius-type temperature dependence. Furthermore, consistency of glass dissolution amount between solid and solution analyses demonstrates the reliability of this test method.
Sumi, Hirotaka; Makigaki, Hikaru; Nakayama, Jiro; Niitsuma, Koichi; Kodaka, Akira
no journal, ,
no abstracts in English