Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yamaguchi, Akiko; Kurihara, Yuichi*; Nagata, Kojiro*; Tanaka, Kazuya; Higaki, Shogo*; Kobayashi, Toru; Tanida, Hajime; Ohara, Yoshiyuki*; Yokoyama, Keiichi; Yaita, Tsuyoshi; et al.
Journal of Colloid and Interface Science, 661, p.317 - 332, 2024/05
Times Cited Count:0no abstracts in English
 bimetallic Ni/Fe
bimetallic Ni/Fe nanoparticles; Implications towards Tc(VII) removal
nanoparticles; Implications towards Tc(VII) removalMaamoun, I.; Tokunaga, Kohei; Dohi, Terumi; Kanno, Futoshi*; Falyouna, O.*; Eljamal, O.*; Tanaka, Kazuya
Frontiers in Nuclear Engineering (Internet), 2, p.1142823_1 - 1142823_17, 2023/03
Recently, the rapid development of nuclear power technologies and the continuous energy demand around the world exhibited massive amounts of contaminated water with radionuclides. Technetium-99 ( Tc) is one of the long-lived radionuclides with a highly mobile anionic form (Tc(VII)) in aqueous solutions. Hence, the rapid and efficient Tc(VII) removal from radioactive water has emerged as a challenging issue. In this work, bimetallic nickel/iron nanoparticles (Ni/Fe
Tc) is one of the long-lived radionuclides with a highly mobile anionic form (Tc(VII)) in aqueous solutions. Hence, the rapid and efficient Tc(VII) removal from radioactive water has emerged as a challenging issue. In this work, bimetallic nickel/iron nanoparticles (Ni/Fe ) were prepared to enhance the immobilization of rhenium (Re(VII)) from aqueous solutions, as the surrogate of Tc(VII).
) were prepared to enhance the immobilization of rhenium (Re(VII)) from aqueous solutions, as the surrogate of Tc(VII).

 -, SeO
-, SeO
 -, and SeO
-, and SeO
 -coprecipitated barite after treatment with phosphate
-coprecipitated barite after treatment with phosphateTokunaga, Kohei; Tanaka, Kazuya; Takahashi, Yoshio*; Kozai, Naofumi
Environmental Science & Technology, 57(8), p.3166 - 3175, 2023/02
Times Cited Count:1 Percentile:52.26(Engineering, Environmental)Coprecipitation of radionuclides with barite has been studied to remove radionuclides from radioactive liquid waste because of its excellent removal efficiency; however, little information exists concerning the stability of the ions coprecipitated with barite. This study systematically investigated the stability of iodate, selenite, and selenate coprecipitated with barite via leaching tests. These oxyanions were gradually leached from the oxyanion-bearing barite into ultrapure water over time. Leaching of the oxyanions significantly increased in leaching solutions containing NaCl (pH5.3), NaNO (pH5.9), and Na
(pH5.9), and Na SO
SO (pH5.7). Conversely, leaching of the oxyanions was suppressed in KH
(pH5.7). Conversely, leaching of the oxyanions was suppressed in KH PO
PO solution (pH8.5), indicating that phosphate stabilized the oxyanion-bearing barite. The effect of phosphate treatment on oxyanion-bearing barite was further investigated. The results showed that the barite surface was modified with phosphate, and a thin surface layer of a barium phosphate-like structure was formed. The amount of oxyanions leached from the phosphate-treated samples into leaching solutions containing NaCl or NaNO
solution (pH8.5), indicating that phosphate stabilized the oxyanion-bearing barite. The effect of phosphate treatment on oxyanion-bearing barite was further investigated. The results showed that the barite surface was modified with phosphate, and a thin surface layer of a barium phosphate-like structure was formed. The amount of oxyanions leached from the phosphate-treated samples into leaching solutions containing NaCl or NaNO was much lower than the amounts leached from the untreated barite samples into ultrapure water. The barite coprecipitation combined with subsequent phosphate treatment may be a promising method to efficiently remove iodate, selenite, and selenate from wastewater and stabilize them as barite coprecipitates.
was much lower than the amounts leached from the untreated barite samples into ultrapure water. The barite coprecipitation combined with subsequent phosphate treatment may be a promising method to efficiently remove iodate, selenite, and selenate from wastewater and stabilize them as barite coprecipitates.
 @Mg(OH)
@Mg(OH) ) into porous media for Cr(VI) removal from groundwater
) into porous media for Cr(VI) removal from groundwaterMaamoun, I.; Falyouna, O.*; Eljamal, R.*; Idham, M. F.*; Tanaka, Kazuya; Eljamal, O.*
Chemical Engineering Journal, 451, Part3, p.138718_1 - 138718_22, 2023/01
Times Cited Count:20 Percentile:87.78(Engineering, Environmental) molecular dynamics simulations reveal the hydration structure of the radium(II) ion
molecular dynamics simulations reveal the hydration structure of the radium(II) ionYamaguchi, Akiko; Nagata, Kojiro*; Kobayashi, Keita; Tanaka, Kazuya; Kobayashi, Toru; Tanida, Hajime; Shimojo, Kojiro; Sekiguchi, Tetsuhiro; Kaneta, Yui; Matsuda, Shohei; et al.
iScience (Internet), 25(8), p.104763_1 - 104763_12, 2022/08
Times Cited Count:9 Percentile:68.46(Multidisciplinary Sciences)no abstracts in English
Maamoun, I.; Bensaida, K.*; Eljamal, R.*; Falyouna, O.*; Tanaka, Kazuya; Tosco, T.*; Sugihara, Yuji*; Eljamal, O.*
Journal of Molecular Liquids, 358, p.119216_1 - 119216_13, 2022/07
Times Cited Count:26 Percentile:98.36(Chemistry, Physical)Maamoun, I.; Falyouna, O.*; Eljamal, R.*; Bensaida, K.*; Tanaka, Kazuya; Tosco, T.*; Sugihara, Yuji*; Eljamal, O.*
Journal of Environmental Chemical Engineering, 10(3), p.107431_1 - 107431_17, 2022/06
Times Cited Count:38 Percentile:97.73(Engineering, Environmental)Shiraishi, Fumito*; Chihara, Ryoji*; Tanimoto, Risa*; Tanaka, Kazuya; Takahashi, Yoshio*
Island Arc, 31(1), p.e12448_1 - e12448_9, 2022/05
Times Cited Count:0 Percentile:0(Geosciences, Multidisciplinary)At Yunotaki Fall in north Japan, manganese-oxidizing bacteria were previously assumed to have oxidized manganese to precipitate birnessite, which relied on oxygen released from algae. However, it remained unclear whether larger-scale manganese oxide precipitation was actually occurring under light conditions. This study evaluated the contribution of indirect oxidation using microelectrodes to analyze local water chemistry, in addition to bulk water chemistry and DNA analyses. The results of this study demonstrate that low bulk pH values in the hot spring water hindered indirect oxidation despite the occurrence of active oxygenic photosynthesis and that direct oxidation by manganese-oxidizing bacteria is considered to dominate in the investigated sample.
Kato, Tomoaki; Kozai, Naofumi; Tanaka, Kazuya; Kaplan, D. I.*; Utsunomiya, Satoshi*; Onuki, Toshihiko
Journal of Nuclear Science and Technology, 59(5), p.580 - 589, 2022/05
Times Cited Count:4 Percentile:56.94(Nuclear Science & Technology)This study reports the effect of fulvic acids, which is a natural organic substance generally contained in groundwater, on the oxidation states of radioactive iodine anions (iodide and iodate). Iodide and iodate are contained in the contaminated water of the Fukushima Daiichi Nuclear Power Plant and supposed to be removed by activated carbon (AC) via adsorption. When fulvic acids does not exist in the experimental system, the adsorption of iodide on AC was less than that of iodate and their oxidation states after the adsorption were not changed. When fulvic acids existed, a fraction of the adsorbed iodate was reduced to iodide. This result indicates that the reduction of the adsorbed iodate progresses during the storage of the spent AC.
Wu, C.*; Tanaka, Kazuya; Tani, Yukinori*; Bi, X.*; Liu, J.*; Yu, Q.*
Science of the Total Environment, 821, p.153265_1 - 153265_9, 2022/05
Times Cited Count:21 Percentile:94.35(Environmental Sciences)Microplastics (MPs) with different particle sizes were co-cultured with a model freshwater fungus, 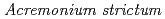 strain KR21-2, to form biofilms on their surface. We also determined the changes in surface physicochemical properties of the biofilm-covered MPs (BMPs) and the heavy metal adsorption capacity of the original MPs and BMPs. The results revealed that the biofilms improve the adsorption of heavy metals on MPs, and the particle size of MPs plays a crucial role in biofilm colonization and adsorption of heavy metals by BMPs.
strain KR21-2, to form biofilms on their surface. We also determined the changes in surface physicochemical properties of the biofilm-covered MPs (BMPs) and the heavy metal adsorption capacity of the original MPs and BMPs. The results revealed that the biofilms improve the adsorption of heavy metals on MPs, and the particle size of MPs plays a crucial role in biofilm colonization and adsorption of heavy metals by BMPs.
Eljamal, O.*; Maamoun, I.; Alkhudhayri, S.*; Eljamal, R.*; Falyouna, O.*; Tanaka, Kazuya; Kozai, Naofumi; Sugihara, Yuji*
Journal of Water Process Engineering (Internet), 46, p.102608_1 - 102608_13, 2022/04
Times Cited Count:30 Percentile:98.51(Engineering, Environmental)Yamaguchi, Akiko; Nagata, Kojiro*; Tanaka, Kazuya; Kobayashi, Keita; Kobayashi, Toru; Shimojo, Kojiro; Tanida, Hajime; Sekiguchi, Tetsuhiro; Kaneta, Yui; Matsuda, Shohei; et al.
Hosha Kagaku, (45), p.28 - 30, 2022/03
no abstracts in English
 /MgO] for prompt and efficient adsorption of ciprofloxacin from aqueous solutions
/MgO] for prompt and efficient adsorption of ciprofloxacin from aqueous solutionsFalyouna, O.*; Bensaida, K.*; Maamoun, I.; Ashik, U. P. M.*; Tahara, Atsushi*; Tanaka, Kazuya; Aoyagi, Noboru; Sugihara, Yuji*; Eljamal, O.*
Journal of Cleaner Production, 342, p.130949_1 - 130949_15, 2022/03
Times Cited Count:41 Percentile:98.81(Green & Sustainable Science & Technology) and Mn
and Mn by fungal manganese oxide through asbolane formation
by fungal manganese oxide through asbolane formationAoshima, Miku*; Tani, Yukinori*; Fujita, Rina*; Tanaka, Kazuya; Miyata, Naoyuki*; Umezawa, Kazuhiro*
Minerals (Internet), 12(3), p.358_1 - 358_16, 2022/03
Times Cited Count:5 Percentile:77.65(Geochemistry & Geophysics)In this study, we conducted sequestration experiments of Co by
by 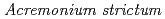 KR21-2 in a Mn
KR21-2 in a Mn /Co
/Co binary solution at pH7. The sequestration of Co
binary solution at pH7. The sequestration of Co by newly formed BMOs readily progressed in parallel with the exogenous Mn
by newly formed BMOs readily progressed in parallel with the exogenous Mn , with higher efficiency than that in single Co
, with higher efficiency than that in single Co solutions. This is attributed to a synergetic effect on Co
solutions. This is attributed to a synergetic effect on Co sequestration through the formation of asbolane in Mn
sequestration through the formation of asbolane in Mn /Co
/Co binary systems.
binary systems.
Tanaka, Kazuya; Tani, Yukinori*; Kozai, Naofumi; Onuki, Toshihiko
Journal of Radioanalytical and Nuclear Chemistry, 331(2), p.1109 - 1114, 2022/02
Times Cited Count:0 Percentile:0.01(Chemistry, Analytical)We investigated the sorption of Pu(IV) on biogenic Mn oxide produced by Mn(II)-oxidizing fungus. The sorption of Pu(IV) on biogenic Mn oxide was similar to that of U(VI) and different from that of Th(IV), possibly due to oxidation of Pu(IV) to Pu(VI). When Pu(IV) was sorbed on hyphae only, it was desorbed into the solution phase over time. Pu(IV) could be solubilized by complexation with organic ligands secreted by fungal cells. In particular, Pu(IV) desorption was observed under circumneutral pH conditions.
Tanaka, Kazuya; Yamasaki, Shinya*
Chikyu Kagaku, 55(4), p.93 - 95, 2021/12
Ten years have passed since the Fukushima Daiichi Nuclear Power Plant (FDNPP) accident. In this special issue, we compiled review papers on the environmental behavior of the FDNPP-derived radionuclides from various research fields. This special issue shows that various research fields contributed to better understandings on the environmental behavior of the FDNPP-derived radionuclides.
 U accumulations in
U accumulations in 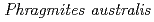 naturally growing at the mill tailings pond; Iron plaque formation possibly related to root-endophytic bacteria producing siderophores
naturally growing at the mill tailings pond; Iron plaque formation possibly related to root-endophytic bacteria producing siderophoresNakamoto, Yukihiro*; Doyama, Kohei*; Haruma, Toshikatsu*; Lu, X.*; Tanaka, Kazuya; Kozai, Naofumi; Fukuyama, Kenjin; Fukushima, Shigeru; Ohara, Yoshiyuki; Yamaji, Keiko*
Minerals (Internet), 11(12), p.1337_1 - 1337_17, 2021/12
Times Cited Count:1 Percentile:10.87(Geochemistry & Geophysics)Mine drainage is a vital water problem in the mining industry worldwide because of the heavy metal elements and low pH. Rhizofiltration using wetland plants is an appropriate method to remove heavy metals from the water via accumulation in the rhizosphere.  is one of the candidate plants for this method because of metal accumulation, forming iron plaque around the roots. At the study site, which was the mill tailings pond in the Ningyo-toge uranium mine,
is one of the candidate plants for this method because of metal accumulation, forming iron plaque around the roots. At the study site, which was the mill tailings pond in the Ningyo-toge uranium mine,  has been naturally growing since 1998. The results showed that
has been naturally growing since 1998. The results showed that  accumulated Fe, Mn, and
accumulated Fe, Mn, and  U in the nodal roots without/with iron plaque compared with other plant tissues. Among the 837 bacterial colonies isolated from nodal roots, 88.6% showed siderophore production activities. Considering iron plaque formation around
U in the nodal roots without/with iron plaque compared with other plant tissues. Among the 837 bacterial colonies isolated from nodal roots, 88.6% showed siderophore production activities. Considering iron plaque formation around  roots, we hypothesized that microbial siderophores might influence iron plaque formation because bacterial siderophores have catechol-like functional groups. The complex of catechol or other phenolics with Fe was precipitated due to the networks between Fe and phenolic derivatives. The experiment using bacterial products of root endophytes, such as
roots, we hypothesized that microbial siderophores might influence iron plaque formation because bacterial siderophores have catechol-like functional groups. The complex of catechol or other phenolics with Fe was precipitated due to the networks between Fe and phenolic derivatives. The experiment using bacterial products of root endophytes, such as 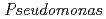 spp. and
spp. and 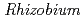 spp., showed precipitation with Fe ions, and we confirmed that several
spp., showed precipitation with Fe ions, and we confirmed that several 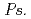 spp. and
spp. and 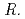 spp. produced unidentified phenolic compounds. In conclusion, root-endophytic bacteria such as
spp. produced unidentified phenolic compounds. In conclusion, root-endophytic bacteria such as 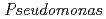 spp. and
spp. and 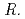 spp., isolated from metal-accumulating roots of
spp., isolated from metal-accumulating roots of 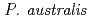 , might influence iron plaque formation as the metal accumulation site. Iron plaque formation is related to tolerance in
, might influence iron plaque formation as the metal accumulation site. Iron plaque formation is related to tolerance in 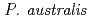 , and
, and 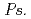 spp. and
spp. and 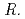 spp. might indirectly contribute to tolerance.
spp. might indirectly contribute to tolerance.
Tokunaga, Kohei; Takahashi, Yoshio*; Tanaka, Kazuya; Kozai, Naofumi
Chemosphere, 266, p.129104_1 - 129104_10, 2021/03
Times Cited Count:10 Percentile:52.13(Environmental Sciences)Radioactive iodine ( I) is of great concern owing to its high mobility in the environment and long-term radiotoxicity, but there is a lack of effective techniques for removing iodate (IO
I) is of great concern owing to its high mobility in the environment and long-term radiotoxicity, but there is a lack of effective techniques for removing iodate (IO
 ) from aqueous solution. The aim of this study is to develop a new technique for removing radioactive iodate from contaminated solution by using barite (BaSO
) from aqueous solution. The aim of this study is to develop a new technique for removing radioactive iodate from contaminated solution by using barite (BaSO ). In the present study, we examined the coprecipitation mechanism of iodate by barite at the molecular level for determining optimum conditions for iodate removal. The results showed that iodate was effectively removed from aqueous solution by coprecipitation, even in the presence of competitive anions in solution. Comparing our method with previous studies, iodate removal efficiency by barite was determined to be about two orders of magnitude greater than that by hydrotalcite-like layered double hydroxide at Cl
). In the present study, we examined the coprecipitation mechanism of iodate by barite at the molecular level for determining optimum conditions for iodate removal. The results showed that iodate was effectively removed from aqueous solution by coprecipitation, even in the presence of competitive anions in solution. Comparing our method with previous studies, iodate removal efficiency by barite was determined to be about two orders of magnitude greater than that by hydrotalcite-like layered double hydroxide at Cl concentration of 10 mmol L
concentration of 10 mmol L . Extended X-ray absorption fine structure (EXAFS) analysis indicated that incorporated iodate was strongly bound in the crystal lattice of barite by substituting the sulfate site in the structure when the iodine concentration was low. The charge compensation problem from the IO
. Extended X-ray absorption fine structure (EXAFS) analysis indicated that incorporated iodate was strongly bound in the crystal lattice of barite by substituting the sulfate site in the structure when the iodine concentration was low. The charge compensation problem from the IO
 substitution in SO
substitution in SO
 site is achieved by the substitution of Na
site is achieved by the substitution of Na -IO
-IO
 pairs at the nearest Ba
pairs at the nearest Ba site. Therefore, considering high removal efficiency and strong binding of iodate in barite, coprecipitation with barite is a promising material for removing radioactive iodate from various aqueous solutions contaminated with iodate.
site. Therefore, considering high removal efficiency and strong binding of iodate in barite, coprecipitation with barite is a promising material for removing radioactive iodate from various aqueous solutions contaminated with iodate.
Tanaka, Kazuya; Kanasashi, Tsutomu*; Takenaka, Chisato*; Takahashi, Yoshio*
Science of the Total Environment, 755(Part 2), p.142598_1 - 142598_8, 2021/02
Times Cited Count:5 Percentile:35.21(Environmental Sciences)In this study, we investigated coordination structures of Cs in  Cs-doped bark, sapwood, heartwood, needle, and branch samples of trees collected in Fukushima by extended X-ray absorption fine structure (EXAFS) spectroscopy. We examined four representative tree species in Fukushima,
Cs-doped bark, sapwood, heartwood, needle, and branch samples of trees collected in Fukushima by extended X-ray absorption fine structure (EXAFS) spectroscopy. We examined four representative tree species in Fukushima, 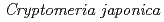 ,
, 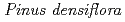 ,
, 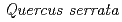 , and
, and 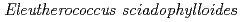 . EXAFS spectra suggested that Cs was adsorbed as an outer-sphere complex on all parts of the four species, with electrostatic binding to negatively charged functional groups in components of tree tissues. These results were supported by extraction experiments where most of the sorbed Cs was desorbed from all parts of each tree species using 1 M CH
. EXAFS spectra suggested that Cs was adsorbed as an outer-sphere complex on all parts of the four species, with electrostatic binding to negatively charged functional groups in components of tree tissues. These results were supported by extraction experiments where most of the sorbed Cs was desorbed from all parts of each tree species using 1 M CH COONH
COONH .
.
 through irreversible biogenic manganese oxide sequestration
through irreversible biogenic manganese oxide sequestrationTani, Yukinori*; Kakinuma, Satomi*; Chang, J.*; Tanaka, Kazuya; Miyata, Naoyuki*
Minerals (Internet), 11(1), p.53_1 - 53_14, 2021/01
Times Cited Count:4 Percentile:45.1(Geochemistry & Geophysics)In this study, we examined the Ba sequestration potential of enzymatically active biogenic Mn oxides (BMOs) with and without exogenous Mn
sequestration potential of enzymatically active biogenic Mn oxides (BMOs) with and without exogenous Mn . The BMOs readily oxidized exogenous Mn
. The BMOs readily oxidized exogenous Mn to produce another BMO phase, and subsequently sequestered Ba
to produce another BMO phase, and subsequently sequestered Ba at a pH of 7.0, with irreversible Ba
at a pH of 7.0, with irreversible Ba sequestration as the dominant pathway. Extended X-ray absorption fine structure spectroscopy and X-ray diffraction analyses demonstrated alteration from turbostratic to tightly stacked birnessite through possible Ba
sequestration as the dominant pathway. Extended X-ray absorption fine structure spectroscopy and X-ray diffraction analyses demonstrated alteration from turbostratic to tightly stacked birnessite through possible Ba incorporation into the interlayer.
incorporation into the interlayer.