Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ishii, Yasuo; Toyoshima, Atsushi; Tsukada, Kazuaki; Asai, Masato; Tome, Hayato; Nishinaka, Ichiro; Nagame, Yuichiro; Miyashita, Sunao*; Mori, Tomotaka*; Suganuma, Hideo*; et al.
Chemistry Letters, 37(3), p.288 - 289, 2008/03
Times Cited Count:20 Percentile:55.03(Chemistry, Multidisciplinary)We have investigated cation-exchange behavior of Rf together with the lighter homologues of the group-4 elements Zr and Hf, and the tetravalent pseudo-homologue Th, in HF/HNO solution using Automated Ion exchange separation apparatus coupled with the Detection system for Alpha spectroscopy (AIDA). The
solution using Automated Ion exchange separation apparatus coupled with the Detection system for Alpha spectroscopy (AIDA). The 
 values of Zr, Hf, Th and Rf in HF/0.1 M HNO
values of Zr, Hf, Th and Rf in HF/0.1 M HNO were decreased with increasing the concentration of the fluoride ion [F
were decreased with increasing the concentration of the fluoride ion [F ], indicating the formation of the fluoride complexes. The sequence of the fluoride complexation strength is Zr
], indicating the formation of the fluoride complexes. The sequence of the fluoride complexation strength is Zr  Hf
Hf  Rf
Rf  Th.
Th.
Haba, Hiromitsu*; Akiyama, Takahiro*; Kaji, Daiya*; Kikunaga, Hidetoshi*; Kuribayashi, Takahiro*; Morimoto, Koji*; Morita, Kosuke*; Oe, Kazuhiro*; Sato, Nozomi*; Shinohara, Atsushi*; et al.
European Physical Journal D, 45(1), p.81 - 86, 2007/10
Times Cited Count:11 Percentile:49.57(Optics)A review is given on the startup of the superheavy element (SHE) chemistry at RIKEN. A gas-jet transport system for the SHE chemistry has been coupled to the gas-filled recoil ion separator GARIS at the RIKEN Linear Accelerator. The performance of the system was appraised using  Fr and
Fr and 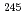 Fm produced in the
Fm produced in the 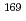 Tm(
Tm(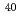 Ar,3
Ar,3 )
) Fr and
Fr and  Pb(
Pb(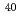 Ar,3
Ar,3 )
)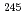 Fm reactions, respectively. The
Fm reactions, respectively. The  particles of
particles of  Fr and
Fr and 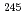 Fm separated with GARIS and transported by the gas-jet were identified with a rotating wheel system for
Fm separated with GARIS and transported by the gas-jet were identified with a rotating wheel system for  spectrometry under desired low background condition. The high gas-jet efficiencies over 80% were independent of the beam intensities up to 2 particle
spectrometry under desired low background condition. The high gas-jet efficiencies over 80% were independent of the beam intensities up to 2 particle  A. A gas-jet coupled target system for the production of SHEs was also installed on the beam line of the RIKEN K70 AVF cyclotron. The gas-jet transport of
A. A gas-jet coupled target system for the production of SHEs was also installed on the beam line of the RIKEN K70 AVF cyclotron. The gas-jet transport of  No and
No and  Rf produced in the
Rf produced in the  U(
U( Ne,5
Ne,5 )
) No and
No and  Cm(
Cm( O,5
O,5 )
) Rf reactions, respectively, was conducted for the future chemical studies of
Rf reactions, respectively, was conducted for the future chemical studies of  Sg via the
Sg via the  Cm(
Cm( Ne, 5
Ne, 5 )
) Sg reaction.
Sg reaction.
Haba, Hiromitsu*; Tsukada, Kazuaki; Asai, Masato; Toyoshima, Atsushi; Ishii, Yasuo; Tome, Hayato; Sato, Tetsuya; Nishinaka, Ichiro; Ichikawa, Takatoshi; Ichikawa, Shinichi; et al.
Radiochimica Acta, 95(1), p.1 - 6, 2007/01
Times Cited Count:15 Percentile:70.27(Chemistry, Inorganic & Nuclear)no abstracts in English
Tsukada, Kazuaki; Toyoshima, Atsushi; Haba, Hiromitsu*; Asai, Masato; Akiyama, Kazuhiko*; Ishii, Yasuo; Tome, Hayato*; Nishinaka, Ichiro; Sato, Tetsuya; Ichikawa, Takatoshi; et al.
no journal, ,
no abstracts in English
Toyoshima, Atsushi; Haba, Hiromitsu*; Tsukada, Kazuaki; Asai, Masato; Akiyama, Kazuhiko*; Ishii, Yasuo; Tome, Hayato*; Nishinaka, Ichiro; Sato, Tetsuya; Ichikawa, Takatoshi; et al.
no journal, ,
no abstracts in English
 OH mixed solution
OH mixed solutionTsukada, Kazuaki; Toyoshima, Atsushi; Haba, Hiromitsu*; Asai, Masato; Akiyama, Kazuhiko*; Ishii, Yasuo; Tome, Hayato; Nishinaka, Ichiro; Sato, Tetsuya; Ichikawa, Shinichi; et al.
no journal, ,
no abstracts in English
 /HF mixed solution, 2
/HF mixed solution, 2Ishii, Yasuo; Tome, Hayato; Toyoshima, Atsushi; Asai, Masato; Nishinaka, Ichiro; Tsukada, Kazuaki; Nagame, Yuichiro; Miyashita, Sunao*; Mori, Tomotaka*; Suganuma, Hideo*; et al.
no journal, ,
Chemical properties of rutherfordium (Rf, the element 104) in HNO /HF mixed solutions have been investigated using cation-exchange chromatography. Distribution coefficients of Rf on the cation-exchange resin as a function of hydrofluoric-acid and nitric-acid concentrations have been measured precisely, and it was found that the strength of the fluoride complex formation of Rf is much weaker than those of lighter group 4 homologs Zr and Hf. The same result was obtained through the anion-exchange chromatography. These results enabled us to quantitatively account for the fluoride complexation of Rf.
/HF mixed solutions have been investigated using cation-exchange chromatography. Distribution coefficients of Rf on the cation-exchange resin as a function of hydrofluoric-acid and nitric-acid concentrations have been measured precisely, and it was found that the strength of the fluoride complex formation of Rf is much weaker than those of lighter group 4 homologs Zr and Hf. The same result was obtained through the anion-exchange chromatography. These results enabled us to quantitatively account for the fluoride complexation of Rf.
 /HF solution
/HF solutionIshii, Yasuo; Tome, Hayato; Toyoshima, Atsushi; Asai, Masato; Nishinaka, Ichiro; Tsukada, Kazuaki; Nagame, Yuichiro; Miyashita, Sunao*; Mori, Tomotaka*; Suganuma, Hideo*; et al.
no journal, ,
The cation-exchange behavior of rutherfordium(Rf) in HNO /HF solution has been studied based on an atom-at-a-time scale. In the symposium, we will discuss the fluoride complexation of rutherfordium in comparison with that of Zr, Hf and Th.
/HF solution has been studied based on an atom-at-a-time scale. In the symposium, we will discuss the fluoride complexation of rutherfordium in comparison with that of Zr, Hf and Th.