Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 MnO
MnO cathode in an all-solid-state thin-film battery during cycling
cathode in an all-solid-state thin-film battery during cyclingHikima, Kazuhiro*; Hinuma, Yoyo*; Shimizu, Keisuke*; Suzuki, Kota*; Taminato, So*; Hirayama, Masaaki*; Masuda, Takuya*; Tamura, Kazuhisa; Kanno, Ryoji*
ACS Applied Materials & Interfaces, 13(6), p.7650 - 7663, 2021/02
Times Cited Count:12 Percentile:67.96(Nanoscience & Nanotechnology)We evaluated the structural change of the cathode material Li MnO
MnO that was deposited as an epitaxial film with an (001) orientation in an all-solid-state battery. In case of the electrode with LiPO
that was deposited as an epitaxial film with an (001) orientation in an all-solid-state battery. In case of the electrode with LiPO coating. Experiments revealed a structural change to a high-capacity (activated) phase that proceeded gradually and continuously with cycling. The activated phase barely showed any capacity fading. We propose a mechanism of structural change with cycling: charging to a high voltage at a sufficiently low Li concentration typically induces irreversible transition to a phase detrimental to cycling that could, but not necessarily, be accompanied by the dissolution of Mn and/or the release of O into the electrolyte, while a gradual irreversible transition to an activated phase happens at a similar Li concentration under a lower voltage.
coating. Experiments revealed a structural change to a high-capacity (activated) phase that proceeded gradually and continuously with cycling. The activated phase barely showed any capacity fading. We propose a mechanism of structural change with cycling: charging to a high voltage at a sufficiently low Li concentration typically induces irreversible transition to a phase detrimental to cycling that could, but not necessarily, be accompanied by the dissolution of Mn and/or the release of O into the electrolyte, while a gradual irreversible transition to an activated phase happens at a similar Li concentration under a lower voltage.
 PO
PO -modified Li
-modified Li RuO
RuO for lithium-rich layered rocksalt oxide cathodes
for lithium-rich layered rocksalt oxide cathodesTaminato, So*; Hirayama, Masaaki*; Suzuki, Kota*; Kim, K.-S.*; Tamura, Kazuhisa; Kanno, Ryoji*
Journal of Physical Chemistry C, 122(29), p.16607 - 16612, 2018/07
Times Cited Count:8 Percentile:31.15(Chemistry, Physical)Lithium-rich layered rocksalt oxides are promising cathode materials for lithium-ion batteries. We investigate the effects of surface modification by amorphous Li PO
PO on the structures and electrochemical reactions in the surface region of an epitaxial Li
on the structures and electrochemical reactions in the surface region of an epitaxial Li RuO
RuO (010) film electrode. Structural characterization using SXRD, HAXPES, and NR shows that surface modification by Li
(010) film electrode. Structural characterization using SXRD, HAXPES, and NR shows that surface modification by Li PO
PO resulted in the partial substitution of P for Li in the surface region of Li
resulted in the partial substitution of P for Li in the surface region of Li RuO
RuO . The modified (010) surface exhibits better rate capability at 20 C compared to the unmodified surface.
. The modified (010) surface exhibits better rate capability at 20 C compared to the unmodified surface. 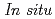 surface XRD confirmed that highly reversible structural changes occurred at the modified surface during lithium (de)intercalation. These results demonstrate that this surface modification stabilizes the crystal structure in the surface region, and it can improve the rate capability of lithium-rich layered rocksalt oxide cathodes.
surface XRD confirmed that highly reversible structural changes occurred at the modified surface during lithium (de)intercalation. These results demonstrate that this surface modification stabilizes the crystal structure in the surface region, and it can improve the rate capability of lithium-rich layered rocksalt oxide cathodes.
 surface under high voltage battery operation
surface under high voltage battery operationTaminato, So*; Hirayama, Masaaki*; Suzuki, Kota*; Tamura, Kazuhisa; Minato, Taketoshi*; Arai, Hajime*; Uchimoto, Yoshiharu*; Ogumi, Zempachi*; Kanno, Ryoji*
Journal of Power Sources, 307, p.599 - 603, 2016/03
Times Cited Count:34 Percentile:71.85(Chemistry, Physical)An epitaxial-film model electrode of LiCoO (104) was fabricated on SrRuO
(104) was fabricated on SrRuO (100)/Nb:SrTiO
(100)/Nb:SrTiO (100) using pulsed laser deposition. The 50 nm thick LiCoO
(100) using pulsed laser deposition. The 50 nm thick LiCoO (104) film exhibited lithium (de-)intercalation activity with a first discharge capacity of 119 mAh g
(104) film exhibited lithium (de-)intercalation activity with a first discharge capacity of 119 mAh g between 3.0 and 4.4 V, followed by a gradual capacity fading with subsequent charge-discharge cycles. In contrast, a 3.2 nm thick Li
between 3.0 and 4.4 V, followed by a gradual capacity fading with subsequent charge-discharge cycles. In contrast, a 3.2 nm thick Li PO
PO -coated film exhibited a higher intercalation capacity of 148 mAh g
-coated film exhibited a higher intercalation capacity of 148 mAh g with superior cycle retention than the uncoated film. In situ surface X-ray diffraction measurements revealed a small lattice change at the coated surface during the (de-)intercalation processes compared to the uncoated surface. The surface modification of LiCoO
with superior cycle retention than the uncoated film. In situ surface X-ray diffraction measurements revealed a small lattice change at the coated surface during the (de-)intercalation processes compared to the uncoated surface. The surface modification of LiCoO by the Li
by the Li PO
PO coating could lead to improvement of the structural stability at the surface region during lithium (de-)intercalation at high voltage.
coating could lead to improvement of the structural stability at the surface region during lithium (de-)intercalation at high voltage.
 O
O epitaxial thin film electrode for lithium batteries
epitaxial thin film electrode for lithium batteriesSuzuki, Kota*; Hirayama, Masaaki*; Kim, K.-S.*; Taminato, So*; Tamura, Kazuhisa; Son, J.-Y.*; Mizuki, Junichiro; Kanno, Ryoji*
Journal of the Electrochemical Society, 162(13), p.A7083 - A7090, 2015/08
Times Cited Count:11 Percentile:36.55(Electrochemistry)The effects of surface coatings on LiMn O
O were investigated using LiMn
were investigated using LiMn O
O epitaxial thin films with a thickness of 30 nm. Bare and surface-coated LiMn
epitaxial thin films with a thickness of 30 nm. Bare and surface-coated LiMn O
O epitaxial thin films were synthesized on SrTiO
epitaxial thin films were synthesized on SrTiO (111) substrates using a pulsed laser deposition method. The surface coating, which was formed using the solid electrolyte Li
(111) substrates using a pulsed laser deposition method. The surface coating, which was formed using the solid electrolyte Li PO
PO and had a thickness of 3 nm, improved the reversibility of the electrochemical reactions undergone by the LiMn
and had a thickness of 3 nm, improved the reversibility of the electrochemical reactions undergone by the LiMn O
O epitaxial thin films. The changes induced in the surface structure were maintained during battery operation; in contrast, the bare LiMn
epitaxial thin films. The changes induced in the surface structure were maintained during battery operation; in contrast, the bare LiMn O
O thin film exhibited structural degradation and Mn dissolution. The structural changes induced in the coated electrode and the increase in its surface stability were intrinsic effects of the Li
thin film exhibited structural degradation and Mn dissolution. The structural changes induced in the coated electrode and the increase in its surface stability were intrinsic effects of the Li PO
PO coating and improved the electrochemical performance of the LiMn
coating and improved the electrochemical performance of the LiMn O
O thin-film electrode.
thin-film electrode.
 RuO
RuO epitaxial film electrodes and
epitaxial film electrodes and 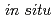 surface X-ray analysis
surface X-ray analysisTaminato, So*; Hirayama, Masaaki*; Suzuki, Kota*; Kim, K.-S.*; Zheng, Y.*; Tamura, Kazuhisa; Mizuki, Junichiro; Kanno, Ryoji*
Journal of Materials Chemistry A, 2(34), p.17875 - 17882, 2014/11
Times Cited Count:21 Percentile:55.49(Chemistry, Physical)The surface structure of a lithium-rich layered material and its relation to intercalation properties were investigated by synchrotron X-ray surface structural analyses using Li RuO
RuO epitaxial-film model electrodes with different lattice planes of (010) and (001). Electrochemical charge-discharge measurements confirmed reversible lithium intercalation activity through both planes, corresponding to three-dimensional lithium diffusion within the Li
epitaxial-film model electrodes with different lattice planes of (010) and (001). Electrochemical charge-discharge measurements confirmed reversible lithium intercalation activity through both planes, corresponding to three-dimensional lithium diffusion within the Li RuO
RuO . The (001) plane exhibited higher discharge capacities compared to the (010) plane under high rate operation (over 5 C). Direct observations of surface structural changes by
. The (001) plane exhibited higher discharge capacities compared to the (010) plane under high rate operation (over 5 C). Direct observations of surface structural changes by 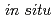 surface X-ray diffraction (XRD) and surface X-ray absorption near edge structure (XANES) established that an irreversible phase change occurs at the (010) surface during the first (de)intercalation process, whereas reversible structural changes take place at the (001) surface.
surface X-ray diffraction (XRD) and surface X-ray absorption near edge structure (XANES) established that an irreversible phase change occurs at the (010) surface during the first (de)intercalation process, whereas reversible structural changes take place at the (001) surface.
 Ti
Ti O
O (110) film electrode for lithium batteries
(110) film electrode for lithium batteriesKim, K.-S.*; Tojigamori, Takeshi*; Suzuki, Kota*; Taminato, So*; Tamura, Kazuhisa; Mizuki, Junichiro; Hirayama, Masaaki*; Kanno, Ryoji*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 80(10), p.800 - 803, 2012/10
Times Cited Count:12 Percentile:29.96(Electrochemistry)Electrochemical properties and structure changes of nano-sized Li Ti
Ti O
O during lithium (de)intercalation wereinvestigated using a two-dimensional thin film electrode. Li
during lithium (de)intercalation wereinvestigated using a two-dimensional thin film electrode. Li Ti
Ti O
O thin films were deposited on a Nb:SrTiO
thin films were deposited on a Nb:SrTiO (110)substrate by a pulsed laser deposition technique. In situ X-ray diffraction measurements clarified the drastic structural changes of the Li
(110)substrate by a pulsed laser deposition technique. In situ X-ray diffraction measurements clarified the drastic structural changes of the Li Ti
Ti O
O film upon soaking in the electrolyte and during the first intercalation and deintercalation processes. The surfaceregion of Li
film upon soaking in the electrolyte and during the first intercalation and deintercalation processes. The surfaceregion of Li Ti
Ti O
O had a different structure from the bulk during electrochemical cycling and could cause the nanosizedLi
had a different structure from the bulk during electrochemical cycling and could cause the nanosizedLi Ti
Ti O
O electrodes to have high capacities and poor stabilities.
electrodes to have high capacities and poor stabilities.