Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
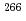 Bh in the
Bh in the  Cm(
Cm(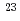 Na,5
Na,5 )
)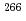 Bh reaction and its decay properties
Bh reaction and its decay propertiesHaba, Hiromitsu*; Fan, F.*; Kaji, Daiya*; Kasamatsu, Yoshitaka*; Kikunaga, Hidetoshi*; Komori, Yukiko*; Kondo, Narumi*; Kudo, Hisaaki*; Morimoto, Koji*; Morita, Kosuke*; et al.
Physical Review C, 102(2), p.024625_1 - 024625_12, 2020/08
Times Cited Count:6 Percentile:58.52(Physics, Nuclear) acidic solutions
acidic solutionsYokoyama, Akihiko*; Kitayama, Yuta*; Fukuda, Yoshiki*; Kikunaga, Hidetoshi*; Murakami, Masashi*; Komori, Yukiko*; Yano, Shinya*; Haba, Hiromitsu*; Tsukada, Kazuaki; Toyoshima, Atsushi*
Radiochimica Acta, 107(1), p.27 - 32, 2019/01
Times Cited Count:1 Percentile:10.81(Chemistry, Inorganic & Nuclear)Tanaka, Taiki*; Narikiyo, Yoshihiro*; Morita, Kosuke*; Fujita, Kunihiro*; Kaji, Daiya*; Morimoto, Koji*; Yamaki, Sayaka*; Wakabayashi, Yasuo*; Tanaka, Kengo*; Takeyama, Mirei*; et al.
Journal of the Physical Society of Japan, 87(1), p.014201_1 - 014201_9, 2018/01
Times Cited Count:18 Percentile:74.14(Physics, Multidisciplinary)Excitation functions of quasielastic scattering cross sections for the  Ca +
Ca +  Pb,
Pb,  Ti +
Ti +  Pb, and
Pb, and  Ca +
Ca +  Cm reactions were successfully measured by using the gas-filled recoil-ion separator GARIS. Fusion barrier distributions were extracted from these data, and compared with the coupled-channels calculations. It was found that the peak energies of the barrier distributions for the
Cm reactions were successfully measured by using the gas-filled recoil-ion separator GARIS. Fusion barrier distributions were extracted from these data, and compared with the coupled-channels calculations. It was found that the peak energies of the barrier distributions for the  Ca +
Ca +  Pb and
Pb and  Ti +
Ti +  Pb systems coincide with those of the 2n evaporation channel cross sections for the systems, while that of the
Pb systems coincide with those of the 2n evaporation channel cross sections for the systems, while that of the  Ca +
Ca +  Cm is located slightly below the 4n evaporation ones. This results provide us helpful information to predict the optimum beam energy to synthesize superheavy nuclei.
Cm is located slightly below the 4n evaporation ones. This results provide us helpful information to predict the optimum beam energy to synthesize superheavy nuclei.
Eichler, R.*; Asai, Masato; Brand, H.*; Chiera, N. M.*; Di Nitto, A.*; Dressler, R.*; D llmann, Ch. E.*; Even, J.*; Fangli, F.*; Goetz, M.*; et al.
llmann, Ch. E.*; Even, J.*; Fangli, F.*; Goetz, M.*; et al.
EPJ Web of Conferences, 131, p.07005_1 - 07005_7, 2016/12
Times Cited Count:3 Percentile:72.98(Chemistry, Inorganic & Nuclear)In recent years gas-phase chemical studies assisted by physical pre-separation allowed for the productions and investigations of fragile single molecular species of superheavy elements. The latest highlight is the formation of very volatile hexacarbonyl compound of element 106, Sg(CO) . Following this success, second-generation experiments were performed to measure the first bond dissociation energy between the central metal atom and the surrounding ligand. The method using a tubular decomposition reactor was developed and successfully applied to short-lived Mo(CO)
. Following this success, second-generation experiments were performed to measure the first bond dissociation energy between the central metal atom and the surrounding ligand. The method using a tubular decomposition reactor was developed and successfully applied to short-lived Mo(CO) , W(CO)
, W(CO) , and Sg(CO)
, and Sg(CO) .
.
Toyoshima, Atsushi; Kanda, Akimitsu*; Ikeda, Takumi*; Yoshimura, Takashi*; Shinohara, Atsushi*; Yano, Shinya*; Komori, Yukiko*; Haba, Hiromitsu*
no journal, ,
Astatine (At) is reported to be bound in a few oxidation states in aqueous solutions. However, their valencies and chemical species are experimentally not identified. In this study, we studied redox behavior of At using redox agents and using a flow electrolytic column.  At was produced in the
At was produced in the 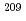 Bi(
Bi( , 2
, 2 )
) At reaction and was then separated from the target by a distillation method. Then, redox of At was carried out in 1.0 M HClO
At reaction and was then separated from the target by a distillation method. Then, redox of At was carried out in 1.0 M HClO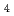 using redox agents or by an electrolysis. After the redox, solvent extraction with HDEHP was performed to identify the redox reactions. Using the redox agents, extraction yields of At were different between the conditions with oxidizing or reducing agents and without redox agents. This means that redox reactions of At can be identified under the present extraction conditions. On the other hand, by the electrolysis, extraction yields of At were almost constant against the variation of applied potential. This is possibly due to the restoration to the original oxidation state after the electrolysis. In the future, we will perform flow electrolytic column chromatography available for simultaneous electrolysis and separation.
using redox agents or by an electrolysis. After the redox, solvent extraction with HDEHP was performed to identify the redox reactions. Using the redox agents, extraction yields of At were different between the conditions with oxidizing or reducing agents and without redox agents. This means that redox reactions of At can be identified under the present extraction conditions. On the other hand, by the electrolysis, extraction yields of At were almost constant against the variation of applied potential. This is possibly due to the restoration to the original oxidation state after the electrolysis. In the future, we will perform flow electrolytic column chromatography available for simultaneous electrolysis and separation.