Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yabe, Makoto; Okamura, Hiroyuki; Ohashi, Akira*; Naganawa, Hirochika; Shimojo, Kojiro
no journal, ,
no abstracts in English
Shimojo, Kojiro; Yabe, Makoto; Okamura, Hiroyuki; Ohashi, Akira*; Naganawa, Hirochika
no journal, ,
no abstracts in English
Yabe, Makoto; Okamura, Hiroyuki; Sairenji, Shiho; Ohashi, Akira*; Naganawa, Hirochika; Shimojo, Kojiro
no journal, ,
no abstracts in English
Yabe, Makoto; Okamura, Hiroyuki; Sairenji, Shiho; Ohashi, Akira*; Naganawa, Hirochika; Shimojo, Kojiro
no journal, ,
no abstracts in English
Yabe, Makoto; Fujiwara, Iori; Okamura, Hiroyuki; Sairenji, Shiho; Ohashi, Akira*; Naganawa, Hirochika; Shimojo, Kojiro
no journal, ,
no abstracts in English
Fujiwara, Iori; Yabe, Makoto; Okamura, Hiroyuki; Oshima, Tatsuya*; Baba, Yoshinari*; Naganawa, Hirochika; Shimojo, Kojiro
no journal, ,
no abstracts in English
Shimojo, Kojiro; Yabe, Makoto*; Sugita, Tsuyoshi; Okamura, Hiroyuki; Ohashi, Akira*; Naganawa, Hirochika
no journal, ,
Solvent extraction is an effective method for the separation and purification of metal ions. The extractant plays a key role in the extraction efficiency and the separation operation. In this study, we have synthesized a novel acidic diamide-type extractant and have comprehensively investigated the extraction of 56 metal ions. It was found that the novel extractant provides excellent extraction performance and separation ability for various rare metal ions such as Sc(III), In(III), Ga(III), and Ni(II) compared with conventional extractants.
Shimojo, Kojiro; Yabe, Makoto*; Sugita, Tsuyoshi; Okamura, Hiroyuki; Ohashi, Akira*; Naganawa, Hirochika
no journal, ,
Solvent extraction is an effective method for the separation and purification of metal ions (e.g. recovery of rare metal and removal of toxic metal). The extractant plays a key role in the extraction efficiency and the separation operation. To date, much effort has been devoted to the development of novel extractants. Most approaches, however, have encountered problems on expensive production cost and low solubility in alkane solvents, and are far from ideal extraction system for practical use. In previous study, we developed inexpensively a diglycolamic acid-type extractant (DODGAA) with monoamide and carboxy group connected by an ether chain, and reported that DODGAA performed better than commercial extractants. In this study, we have synthesized a novel acidic diamide-type extractant (TONTADA) with diamide and carboxy group connected by a nitrogen donor, and have comprehensively investigated the extraction performance. It was found that the TONTADA selectively extracts valuable metal ions such as Cu(II), Ni(II), and Co(II) from base metal ions compared with DODGAA.
Shimojo, Kojiro; Sugita, Tsuyoshi; Yabe, Makoto*; Okamura, Hiroyuki; Ohashi, Akira*; Naganawa, Hirochika
no journal, ,
Solvent extraction is an effective method for the separation and purification of metal ions. The extractant plays a key role in the extraction efficiency and the separation operation. In this study, we have synthesized a novel acidic diamide-type extractant and have comprehensively investigated the extraction of 56 metal ions. It was found that the novel extractant provides excellent extraction performance and separation ability for various rare metal ions such as Sc(III), In(III), Ga(III), and Ni(II) compared with conventional extractants.
Shimojo, Kojiro; Sugita, Tsuyoshi; Yabe, Makoto*; Okamura, Hiroyuki; Ohashi, Akira*; Naganawa, Hirochika
no journal, ,
Solvent extraction is an effective method for the separation and purification of metal ions. In previous study, we developed dioctyldiglycolamic acid extractant (DODGAA) with an amide group and a carboxy group connected by an ether chain, and reported DODGAA performs better than commercial extractants. In this study, we have synthesized a novel acidic diamide-type extractant (TONTADA) with two amide groups and a carboxy group connected by a nitrogen donor atom and have comprehensively investigated the extraction of 56 metal ions. It was found that the novel extractant TONTADA provides excellent extraction performance and separation ability for various rare metal ions such as Sc(III), Cu(II), Ni(II), Co(II), In(III), Ga(III), Hg(II), platinum-group metal, Hf(IV), V(V), Mo, W(VI), and Re(VII) compared with conventional extractants and DODGAA.
Shimojo, Kojiro; Yabe, Makoto*; Sugita, Tsuyoshi; Okamura, Hiroyuki; Ohashi, Akira*; Naganawa, Hirochika
no journal, ,
Solvent extraction is an effective method for the separation and purification of metal ions. The extractant plays a key role in the extraction efficiency and the separation operation. In this study, we have synthesized calix[4]arene diglycolamic acid derivative as a novel extractant and have investigated extraction behavior of lanthanide ions. It was found that the pre-organization of four diglycolamic acid groups in the cyclic structure can improve remarkably extraction performance and separation ability for lanthanide ions.
Shimojo, Kojiro; Yabe, Makoto*; Ikeda, Mari*; Hirayama, Naoki*; Habata, Yoichi*
no journal, ,
Indium(In) and gallium(Ga) are important elements used in displays and semiconductors. However, In and Ga are extremely rare metals that are contained in trace amounts in zinc(Zn) mines. A technology for selectively separating and recovering trace amounts of In and Ga from Zn mines has been desired. We developed a new extractant, nitriloacetic acid diamide ligand (TONAADA), and investigated the extraction and separation of In and Ga from a simulated Zn mines (a mixed aqueous solution of Zn, Al, In, and Ga). As a result, the high selectivity of TONAADA was shown in the order of In  Ga
Ga 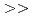 Zn
Zn  Al, and it was clarified that In and Ga can be separated and recovered from Zn and Al in a single extraction. In addition, single-crystal X-ray diffraction demonstrated that In and TONAADA form a 1:2 complex.
Al, and it was clarified that In and Ga can be separated and recovered from Zn and Al in a single extraction. In addition, single-crystal X-ray diffraction demonstrated that In and TONAADA form a 1:2 complex.
Shimojo, Kojiro; Yabe, Makoto*; Ikeda, Mari*; Hirayama, Naoki*; Habata, Yoichi*
no journal, ,
Indium(In) and gallium(Ga) are important elements used in displays and semiconductors. However, In and Ga are extremely rare metals that are contained in trace amounts in zinc(Zn) mines. A technology for selectively separating and recovering trace amounts of In and Ga from Zn mines has been desired. We developed a new extractant, nitriloacetic acid diamide ligand (TONAADA), and investigated the extraction and separation of In and Ga from a simulated Zn mines (a mixed aqueous solution of Zn, Al, In, and Ga). As a result, the high selectivity of TONAADA was shown in the order of In  Ga
Ga  Zn = Al, and it was clarified that In and Ga can be separated and recovered from Zn and Al in a single extraction. In addition, single-crystal X-ray diffraction demonstrated that In and TONAADA form a 1:2 complex.
Zn = Al, and it was clarified that In and Ga can be separated and recovered from Zn and Al in a single extraction. In addition, single-crystal X-ray diffraction demonstrated that In and TONAADA form a 1:2 complex.