Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sakashita, Koichi; Ishii, Naoyuki; Kijima, Jun; Aoyagi, Yoshitaka; Hagiwara, Masayoshi; Fukushima, Mineo
JAEA-Testing 2020-003, 20 Pages, 2020/07
Steam reforming method has been developed for the treatment of organic wastes which are not suitable materials (halogenated oil) for the incineration. This method consists of the gasification process in which organics are vaporized and decomposed with superheated steam and the oxidation process in which vaporized organics are decomposed by oxidizing reaction with heated air. In the gasification process, nonvolatile radionuclides are separated from vaporized waste. Therefore it can be expected that treatment of liquid waste generated from an off-gas treatment system and maintenance operation of the off-gas treatment system become easy to perform. 1,500L of waste oil contaminated with halogen, solvent and uranium was treated using the demonstration scale steam reforming system to examine the performance of the system in 2018. Results obtained this study were as follows; (1) The temperature in the steam reforming system was controlled under the self-regulation temperature. (2) The concentration of CO and NO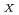 in the off-gas were controlled less than 100 ppm and 250 ppm respectively. (3) The gasification ratio of waste oil was more than 99%. (4) Concentration of fluorine oil in waste oil is needed to be less than 20wt% to perform stable continuance treatment.
in the off-gas were controlled less than 100 ppm and 250 ppm respectively. (3) The gasification ratio of waste oil was more than 99%. (4) Concentration of fluorine oil in waste oil is needed to be less than 20wt% to perform stable continuance treatment.
Okadome, Yoshihiro; Aoyama, Yoshio; Sasaki, Yu; Fukushima, Mineo
Proceedings of 2017 International Congress on Advances in Nuclear Power Plants (ICAPP 2017) (CD-ROM), 6 Pages, 2017/04
 -eyeII
-eyeIIKanayama, Fumihiko; Okada, Takashi; Fukushima, Mineo; Yoshimoto, Katsunobu*; Hanyu, Toshinori; Kawanobe, Takayuki
JAEA-Technology 2013-049, 60 Pages, 2014/03
For planning of removing fuels and debris from the Unit 2 reactor building, TEPCO has already started to measure dose rate over the floor by remotely operated vehicle. Because the measured data were widely distributed in the range of several decades to one thousand mSv/h, it is necessary for TEPCO to survey of contamination distribution on operation floor 2 for more detail planning. JAEA estimated sensitivity of developed gamma camera system named " -eye II" in consistency with actual radiation condition, and carried a demonstration experiment at Fukushima Daiichi N.P.P. to confirm a strength of jamming by back ground dose. Then, JAEA surveyed contamination distribution of operating floor using
-eye II" in consistency with actual radiation condition, and carried a demonstration experiment at Fukushima Daiichi N.P.P. to confirm a strength of jamming by back ground dose. Then, JAEA surveyed contamination distribution of operating floor using  -eye II. At the result of survey, it was found that, - main radiation source in survey area was located on upper reactor well, - western floor in survey area was lower the margin of capacity of
-eye II. At the result of survey, it was found that, - main radiation source in survey area was located on upper reactor well, - western floor in survey area was lower the margin of capacity of  -eye II, -there was a highly contaminated spot on the floor near the opened BOP.
-eye II, -there was a highly contaminated spot on the floor near the opened BOP.
 system using a combination of X-ray diffraction and differential thermal analyses
system using a combination of X-ray diffraction and differential thermal analysesNakayoshi, Akira; Kitawaki, Shinichi; Fukushima, Mineo; Murakami, Tsuyoshi*; Kurata, Masaki
Journal of Nuclear Materials, 441(1-3), p.468 - 472, 2013/10
Times Cited Count:14 Percentile:72.14(Materials Science, Multidisciplinary)Electrorefining is one of the main steps of pyroreprocessing where spent nuclear fuels are recycled. Electrorefining is conducted in a molten salt of LiCl-KCl eutectic (59:41 mol%) containing actinide chlorides (AnCl ) at 773 K. In order to operate and maintain the electrorefiner, it is necessary to accumulate fundamental data on LiCl-KCl-AnCl
) at 773 K. In order to operate and maintain the electrorefiner, it is necessary to accumulate fundamental data on LiCl-KCl-AnCl salt such as the melting point. In this study, based on X-ray diffraction and differential thermal analysis, a partial phase diagram of (LiCl-KCl)eut.-UCl
salt such as the melting point. In this study, based on X-ray diffraction and differential thermal analysis, a partial phase diagram of (LiCl-KCl)eut.-UCl pseudo-binary system and partial phase diagram of LiCl-KCl-UCl
pseudo-binary system and partial phase diagram of LiCl-KCl-UCl system were developed, which UCl
system were developed, which UCl concentration was up to 20 mol%.
concentration was up to 20 mol%.
Maeda, Koji; Sasaki, Shinji; Kumai, Misaki; Sato, Isamu; Osaka, Masahiko; Fukushima, Mineo; Kawatsuma, Shinji; Goto, Tetsuo*; Sakai, Hitoshi*; Chigira, Takayuki*; et al.
Proceedings of International Nuclear Fuel Cycle Conference; Nuclear Energy at a Crossroads (GLOBAL 2013) (CD-ROM), p.272 - 277, 2013/09
Fukushima, Mineo; Kawatsuma, Shinji; Okada, Takashi
Proceedings of American Nuclear Society Embedded Topical on Decommissioning, Decontamination and Reutilization and Technology Expo (DD&R 2012) (DVD-ROM), p.67 - 68, 2012/06
The accident on Fukushima Daiichi Nuclear Power Plant has been occurred by the TSUNAMI that generated form Great East Japan Earthquake happened in 11th March 2011. Just after the earthquake, JAEA is assisting activities concerning the accident of the Fukushima No.1 Nuclear Power Station including teleoperation, decontamination and radiation monitoring in the site. JAEA had already developed some robotics, RESQ series, for radiological emergency response in 2001, after JCO criticality accidents occurred. However, they could not work for the NPP because of lack of maintenance. According to the situation and condition of the FUKUSHIMA-DAIICHI accident, JAEA has modified above mentioned robotics and prepared supporting equipments like as Robotics control vehicles. JAEA has provided Robotics and Robotics Control vehicles to TEPCO and is continuously supporting Tokyo Electric Company for plant restoration.
Kawatsuma, Shinji; Fukushima, Mineo; Okada, Takashi
Industrial Robot; An International Journal, 39(5), p.428 - 435, 2012/00
Times Cited Count:120 Percentile:95.92(Engineering, Industrial) C
CNagai, Takayuki; Uehara, Akihiro*; Fukushima, Mineo; Myochin, Munetaka; Fujii, Toshiyuki*; Sato, Nobuaki*; Yamana, Hajimu*
Proceedings in Radiochemistry, 1(1), p.151 - 155, 2011/09
Absorption spectra of dissolved uranium species in molten Li MoO
MoO -Na
-Na MoO
MoO eutectic at 550
eutectic at 550  C were measured by spectrophotometry, and their redox reactions were also investigated by cyclic voltammetry. Observed absorption spectra of uranium species were similar to those of UO
C were measured by spectrophotometry, and their redox reactions were also investigated by cyclic voltammetry. Observed absorption spectra of uranium species were similar to those of UO
 in molten chlorides. After purging oxygen into the melt, the absorption peaks of UO
in molten chlorides. After purging oxygen into the melt, the absorption peaks of UO
 decreased and UO
decreased and UO
 was thought to be oxidized to UO
was thought to be oxidized to UO
 . When the uranium species were not contained in the melt, we confirmed that alkali metals deposited at -0.7 V and a small reduction of this melt was observed at -0.3 V. When UO
. When the uranium species were not contained in the melt, we confirmed that alkali metals deposited at -0.7 V and a small reduction of this melt was observed at -0.3 V. When UO was dissolved into the melt, the reduction of the uranium species was observed at -0.2 V. It was suggested that the dissolved uranium species are recovered as mixed uranium-molybdenum oxides by electrolysis.
was dissolved into the melt, the reduction of the uranium species was observed at -0.2 V. It was suggested that the dissolved uranium species are recovered as mixed uranium-molybdenum oxides by electrolysis.
Kitawaki, Shinichi; Nakayoshi, Akira; Fukushima, Mineo; Sakamura, Yoshiharu*; Murakami, Tsuyoshi*; Akiyama, Naoyuki*
Proceedings of International Conference on Toward and Over the Fukushima Daiichi Accident (GLOBAL 2011) (CD-ROM), 5 Pages, 2011/09
In the FaCT project, the metal fuel cycle including metal fuel fast reactor and pyrochemical reprocessing has been being developed. JAEA and CRIEPI have continued a collaborative study on pyrochemical reprocessing. In the pyrochemical reprocessing, actinides in the spent fuels dissolve anodically in the LiCl-KCl, and U is collected selectively on a solid cathode, Pu and MA are recovered simultaneously in a liquid Cd cathode. In the previous electrorefining tests, at the anode Zr was allowed to dissolve into the electrolyte salt together with U, Pu and MA. The Zr co-dissolution may cause some problems. In this study, through the anode dissolution test of U-Pu-Zr alloy fuel, the controlling the dissolution of the Zr and the improvement of dissolution ratio of U, Pu were studied. The U-Pu alloy was prepared from MOX pellets by using the electrochemical reduction method. U-Pu-Zr ternary alloy was produced by alloying the obtained U-Pu alloy and prepared U-Zr alloy. U-Pu-Zr ternary alloy was immersed into electrolyte salt, and electrolysis test was carried out.
Murakami, Tsuyoshi*; Sakamura, Yoshiharu*; Akiyama, Naoyuki*; Kitawaki, Shinichi; Nakayoshi, Akira; Fukushima, Mineo
Journal of Nuclear Materials, 414(2), p.194 - 199, 2011/07
Times Cited Count:17 Percentile:77.43(Materials Science, Multidisciplinary)An electrorefining is one of the main steps of pyrochemical reprocessing of spent metallic fuels (U-Zr, U-Pu-Zr). The electrorefining is carried out dissolving a portion of Zr together with actinides to accomplish a high dissolution ratio of actinides. However, the electrorefining with Zr co-dissolution should bring some practical problems in the pyrochemical reprocessing. Therefore, electrorefining tests of non-irradiated U-Pu-Zr alloy were performed with minimizing the amount of Zr dissolved in LiCl-KCl-(U, Pu, Am)Cl melts at 773 K. The tests were performed both by potentiostatic electrolysis at -1.0 V (Ag
melts at 773 K. The tests were performed both by potentiostatic electrolysis at -1.0 V (Ag /Ag) that was more negative than the Zr dissolution potential and by galvanostatic electrolysis with a limited amount of Zr dissolution. The ICP-AES analysis of the anode residues confirmed that a high dissolution ratio of actinides (U;
/Ag) that was more negative than the Zr dissolution potential and by galvanostatic electrolysis with a limited amount of Zr dissolution. The ICP-AES analysis of the anode residues confirmed that a high dissolution ratio of actinides (U;  99.6%, Pu; 99.9%) was successfully demonstrated at both electrolyses.
99.6%, Pu; 99.9%) was successfully demonstrated at both electrolyses.
Kofuji, Hirohide; Fukushima, Mineo; Kitawaki, Shinichi; Myochin, Munetaka; Kormilitsyn, M. V.*; Terai, Takayuki*
IOP Conference Series; Materials Science and Engineering, 9, p.012010_1 - 012010_8, 2010/05
Times Cited Count:0 Percentile:1.02(Chemistry, Inorganic & Nuclear)Kitawaki, Shinichi; Nakayoshi, Akira; Fukushima, Mineo; Koizumi, Tsutomu; Kurata, Masaki*; Yahagi, Noboru*
Proceedings of International Conference on Advanced Nuclear Fuel Cycle; Sustainable Options & Industrial Perspectives (Global 2009) (CD-ROM), p.1269 - 1273, 2009/09
JAEA is developing the pyroreprocessing by collaboration with CRIEPI. The test using U began in 2002, and the test using PuO and unirradiated MOX were ended in 2008. The reduction of UO
and unirradiated MOX were ended in 2008. The reduction of UO pellets by using Li-reduction method, the electrowinning using reduced pellets, the separation of adhered salt with deposit by distillation, and the ingot formation of deposit were performed. As a result, 99% of the loaded U is recovered as metal ingot. The tests similar to U tests were performed by using PuO
pellets by using Li-reduction method, the electrowinning using reduced pellets, the separation of adhered salt with deposit by distillation, and the ingot formation of deposit were performed. As a result, 99% of the loaded U is recovered as metal ingot. The tests similar to U tests were performed by using PuO . As a result, Pu was successfully recovered with U metal. In the MOX test, the mass balance of Pu was maintained at
. As a result, Pu was successfully recovered with U metal. In the MOX test, the mass balance of Pu was maintained at  100% with respect to the initial amount. We try to form the U-Pu-Zr alloy by using reduced MOX. After 2009, the process development that uses the alloy will be continued.
100% with respect to the initial amount. We try to form the U-Pu-Zr alloy by using reduced MOX. After 2009, the process development that uses the alloy will be continued.
Nagai, Takayuki; Kikuchi, Kotaro*; Kano, Yoshiharu*; Fukushima, Mineo
Nihon Genshiryoku Gakkai Wabun Rombunshi, 7(4), p.370 - 379, 2008/12
Study of a new pyrochemical processing using alkaline molybdate melt has been carrying out as a candidate reprocessing process for spent oxide fuels. In our previous study, we had confirmed that melting of UO pellet into a molten Na
pellet into a molten Na MoO
MoO -MoO
-MoO and electrolytic recovering of UO
and electrolytic recovering of UO from the melt included uranyl ions. In this report, the corrosion behavior of the SUS316 cladding tube in molten Na
from the melt included uranyl ions. In this report, the corrosion behavior of the SUS316 cladding tube in molten Na MoO
MoO -MoO
-MoO was evaluated, and we confirmed the dissolution of SUS316 elements into the melt when the amount of additional MoO
was evaluated, and we confirmed the dissolution of SUS316 elements into the melt when the amount of additional MoO was excess. And, it is necessary to understand an appropriate amount of additional MoO
was excess. And, it is necessary to understand an appropriate amount of additional MoO since the result of this study.
since the result of this study.
Kitawaki, Shinichi; Shinozaki, Tadahiro; Fukushima, Mineo; Usami, Tsuyoshi*; Yahagi, Noboru*; Kurata, Masaki*
Nuclear Technology, 162(2), p.118 - 123, 2008/05
Times Cited Count:18 Percentile:74.54(Nuclear Science & Technology)A series test of pyro-process was carried out to recover U-Pu alloy from MONJU MOX pellets. In the Li-reduction step, the reduction behavior of MOX was similar to that of UO . In the electrorefining step, the separation factor between U and Pu was 5.7 for the combination of reduced MOX anode and liquid cadmium cathode, which is almost comparable to the value in the previous studies. For the material balance, approximately 98% of U and 103% of Pu were detected in the electrodes or molten salt after the electrolysis with respect to the initial amounts in the anode or molten salt. Considering the analytical error of ICP-AES, these values are reasonable. The remained amount of U in the anode was slightly larger than that of Pu due to the re-oxidation. The U-Pu alloy ingot was successfully formed by distillation of Cd.
. In the electrorefining step, the separation factor between U and Pu was 5.7 for the combination of reduced MOX anode and liquid cadmium cathode, which is almost comparable to the value in the previous studies. For the material balance, approximately 98% of U and 103% of Pu were detected in the electrodes or molten salt after the electrolysis with respect to the initial amounts in the anode or molten salt. Considering the analytical error of ICP-AES, these values are reasonable. The remained amount of U in the anode was slightly larger than that of Pu due to the re-oxidation. The U-Pu alloy ingot was successfully formed by distillation of Cd.
 -UCl
-UCl melt
meltFukushima, Mineo; Nakayoshi, Akira; Kitawaki, Shinichi; Kurata, Masaki*; Yahagi, Noboru*
Proceedings of 3rd International ATALANTE Conference (ATALANTE 2008) (CD-ROM), 4 Pages, 2008/05
Products on solid cathodes recovered from metal pyrochemical processing were processed to obtain uranium ingot. Studies on process conditions for uranium forming, assay recovered uranium products and by-products and evaluation of mass balance were carried out. In this tests, it is confirmed that uranium ingots can be obtained with heating the products up to more than melting temperature of metal uranium under normal pressure because adhered salt cover the uranium not to oxidize it during uranium cohering. Volatilization of americium is very small under the condition of high temperature.
 MoO
MoO melt
meltMizuguchi, Koji; Yasuike, Yoshiyuki*; Fukushima, Mineo; Myochin, Munetaka
Nihon Genshiryoku Gakkai Wabun Rombunshi, 6(4), p.484 - 490, 2007/12
We develop a new decladding process which integrates with dissolution using the molybdenum oxide melt. This melt possesses characteristics that can make actinide oxide dissolve at high speed without reacting with metal such as the cladding tube. To construct the new process, it is necessary to clarify the reaction mechanisms of uranium in dissolution, oxidation, and electrolysis in this melt. In this study, the gram-scale uranium examination was carried out, and the reaction mechanism of the uranium in the melt was clarified. In Na MoO
MoO - Na
- Na Mo
Mo O
O melts, uranium dioxide dissolves as Na
melts, uranium dioxide dissolves as Na U(MoO
U(MoO )
) without changing its oxidation number. In the oxidation, uranium valence was oxidized from IV to VI with oxygen. In electrolysis process, it was clarified that the low current efficiency was caused by the re-dissolution of deposit. The granulated UO
without changing its oxidation number. In the oxidation, uranium valence was oxidized from IV to VI with oxygen. In electrolysis process, it was clarified that the low current efficiency was caused by the re-dissolution of deposit. The granulated UO was recovered on the cathode by adjusting the temperature of the melt to 700 degrees centigrade at which re-dissolution was reduced.
was recovered on the cathode by adjusting the temperature of the melt to 700 degrees centigrade at which re-dissolution was reduced.
Nakayoshi, Akira; Kitawaki, Shinichi; Fukushima, Mineo; Kurata, Masaki*; Yahagi, Noboru*
Proceedings of International Symposium on EcoTopia Science 2007 (ISETS '07) (CD-ROM), p.1062 - 1066, 2007/11
Pyro-reprocessing is one of the promising reprocessing methods for recycling spent nuclear fuels generated from fast reactors. Comparing to the conventional aqueous-processes, following benefits are expected when introducing the pyro-reprocessing, such as reduction of environmental burden, enhancement of proliferation-resistant, enhancement of economical potential, efficient utilization of nuclear resources. The pyro-reprocessing will therefore become more attractive not only in developed countries regarding nuclear energy, but also in developing countries. As for reducing environmental burden, the most important subject is establishment of the nuclear fuel cycle, in which actinide elements are closed. Various kinds of intermediate waste which contains actinide elements are formed in the practical operation not only from the main steps of the pyro-reprocessing but also from related sub-streams.
Koyama, Tadafumi*; Hijikata, Takatoshi*; Usami, Tsuyoshi*; Inoue, Tadashi*; Kitawaki, Shinichi; Shinozaki, Tadahiro; Fukushima, Mineo; Myochin, Munetaka
Journal of Nuclear Science and Technology, 44(3), p.382 - 392, 2007/03
Times Cited Count:24 Percentile:82.59(Nuclear Science & Technology)Electrometallurgical pyroprocessing is a promising technology to realize actinide fuel cycle. Integrated experiments to demonstrate electrometallurgical pyroprocessing of plutonium oxide in continuous operation were carried out. In each test, 10 to 20 g of PuO was reacted with Li reductant to form metal product. The reduction products were charged in the anode basket of the electrorefiner with LiCl-KCl-UCl
was reacted with Li reductant to form metal product. The reduction products were charged in the anode basket of the electrorefiner with LiCl-KCl-UCl electrolyte. With using the anodes, deposition of uranium on the solid cathode was carried out, when PuCl
electrolyte. With using the anodes, deposition of uranium on the solid cathode was carried out, when PuCl concentration was low. After Pu/U ratio in salt electrolyte was increased enough, plutonium and uranium were recovered simultaneously on the liquid cadmium cathode. By heating up the deposits for distillation of the salt and the cadmium, U metal or Pu-U alloyed metal were obtained as residues in the crucible. It was first result to demonstrate the recovery of metal actinides in the continuous operation of pyroprocessing of oxide fuels.
concentration was low. After Pu/U ratio in salt electrolyte was increased enough, plutonium and uranium were recovered simultaneously on the liquid cadmium cathode. By heating up the deposits for distillation of the salt and the cadmium, U metal or Pu-U alloyed metal were obtained as residues in the crucible. It was first result to demonstrate the recovery of metal actinides in the continuous operation of pyroprocessing of oxide fuels.
Fukushima, Mineo; Kobayashi, Tsuguyuki; Myochin, Munetaka; Keiiji, Fujii,*
Journal of Nuclear Science and Technology, 42(10), p.861 - 868, 2005/10
Times Cited Count:5 Percentile:35.8(Nuclear Science & Technology)Focusing on the cover layer materials (as the Radon Barrier Materials), which could have the effect to restrain the radon from scattering into the air and the effect of the radiation shielding, we produced the radon barrier materials with crude bentonite on an experimental basis, using the rotary type comprehensive unit for grinding and mixing, through which we carried out the evaluation of the characteristics thereof.
Kitawaki, Shininchi; Shinozaki, Tadahiro; Fukushima, Mineo; Hijikata, Takatoshi*; Usami, Tsuyoshi*; Koyama, Tadafumi*
Proceedings of International Conference on Nuclear Energy System for Future Generation and Global Sustainability (GLOBAL 2005) (CD-ROM), 5 Pages, 2005/10
Electrometallurgical pyroprocess is a key innovative technology to realize closed actinides cycle with keeping high proliferation-resistance and economy. JNC and CRIEPI have been carrying out the integrated tests of electrometallurgical reprocessing of metal and oxide Pu-containing fuel to demonstrate whole process in continuous operation. After intensive cold test to confirm the functions of experimental apparatus and the test conditions, recovery test of actinide metal product from actinide oxide fuels has started as a first series of the integrated tests. In this report, result of the recovery test is described to elucidate the material flow in the process.