Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Fukuda, Tatsuya*; Takahashi, Ryo*; Hara, Takuhi*; Ohara, Koji*; Kato, Kazuo*; Matsumura, Daiju; Inaba, Yusuke*; Nakase, Masahiko*; Takeshita, Kenji*
Journal of Nuclear Science and Technology, 58(4), p.399 - 404, 2021/04
Times Cited Count:6 Percentile:60.71(Nuclear Science & Technology)Yin, X.; Zhang, L.*; Meng, C.*; Inaba, Yusuke*; Wang, X.*; Nitta, Ayako; Koma, Yoshikazu; Takeshita, Kenji*
Journal of Hazardous Materials, 387, p.121677_1 - 121677_10, 2020/04
Times Cited Count:12 Percentile:54.1(Engineering, Environmental)Tsutsui, Nao; Ban, Yasutoshi; Suzuki, Hideya*; Nakase, Masahiko*; Ito, Sayumi*; Inaba, Yusuke*; Matsumura, Tatsuro; Takeshita, Kenji*
Analytical Sciences, 36(2), p.241 - 246, 2020/02
Times Cited Count:21 Percentile:79.8(Chemistry, Analytical)To investigate the effective separation of actinides (Ans) from lanthanides (Lns), single-stage batch extraction experiments were performed with a novel extractant, tetradodecyl-1,10-phenanthroline-2,9-diamide (TDdPTDA) with various diluents such as 3-nitrobenzotrifluoride (F-3), nitrobenzene, and  -dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from
-dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from  -dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
-dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
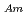 ) and Cm (
) and Cm (
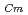 ) for TDdPTDA, with the high separation factors (
) for TDdPTDA, with the high separation factors ( s) of Am from Lns enough for their separation.
s) of Am from Lns enough for their separation.
Mishima, Ria; Inaba, Yusuke*; Tachioka, Sotaro*; Harigai, Miki*; Watanabe, Shinta*; Onoe, Jun*; Nakase, Masahiko*; Matsumura, Tatsuro; Takeshita, Kenji*
Chemistry Letters, 49(1), p.83 - 86, 2020/01
Times Cited Count:4 Percentile:21.18(Chemistry, Multidisciplinary)Separation of platinum group metals (PGMs) from high-level liquid waste generated from the reprocessing of spent nuclear fuels is important to produce good quality vitrified glass for final disposal. A new sorbent, Aluminum hexacyanoferrate (AlHCF), was synthesized and the general sorption behavior of PGMs from concentrated nitric acid was examined. Nitric acid caused substantial elution of AlHCF but the sorption of Pd stabilized the structure. Consequently, Rh was sorbed in the presence of Pd, whereas single Rh sorption caused complete dissolution of AlHCF. Relation between sorbed mount of Pd vs eluted Al and Fe revealed that the elution ratio of Al and Fe was not the same as molar ratio of synthesized AlHCF, indicating the re-sorption of Fe resulted in formation of new structure. The sorption mechanism of PGMs by this new sorbent, AlHCF, not only the simple ion exchange, but also oxidation reduction reaction as well as kinetics play important rule. Understanding the general sorption and dissolution behavior will help improve the sorption performance of PGMs by AlHCF.
Onishi, Takashi; Sekioka, Ken*; Suto, Mitsuo*; Tanaka, Kosuke; Koyama, Shinichi; Inaba, Yusuke*; Takahashi, Hideharu*; Harigai, Miki*; Takeshita, Kenji*
Energy Procedia, 131, p.151 - 156, 2017/12
Times Cited Count:11 Percentile:98.3(Energy & Fuels)no abstracts in English
Koyama, Shinichi; Suto, Mitsuo; Obayashi, Hiroshi; Takeshita, Kenji*; Ogata, Takeshi*; Oaki, Hiroshi*; Inaba, Yusuke*
Separation Science and Technology, 47(14-15), p.2024 - 2028, 2012/11
Times Cited Count:3 Percentile:15.89(Chemistry, Multidisciplinary)A series of separation experiments was performed to study the recovery process for minor actinides (MAs) from the actual spent fuel by using an extraction chromatographic technique. TPPEN gel was used as a new extraction chromatographic agent. Mixed oxide fuel was used as a reference spent fuel to demonstrate the recovery of the MAs. The MOX fuel, including 29.9 wt% plutonium (Pu), was irradiated up to 119 GWd/MTM, and the fuel was then prepared for the extraction experiment. A Mixed solution of MAs and lanthanides (Lns) was prepared. The TPPEN gel was immersed in a 0.01 M NaNO solution, and the pH was adjusted to 4.0. Next, an extraction column was prepared using the TPPEN gel, and the mixed solution of MAs and Lns was passed through the column. The Lns were detected in the eluent after washing with 0.01 M NaNO
solution, and the pH was adjusted to 4.0. Next, an extraction column was prepared using the TPPEN gel, and the mixed solution of MAs and Lns was passed through the column. The Lns were detected in the eluent after washing with 0.01 M NaNO (pH 4.0). For detecting the MAs, the pH of the eluent was changed to 2.0.
(pH 4.0). For detecting the MAs, the pH of the eluent was changed to 2.0.
Inaba, Yusuke*; Tsumagari, Takayuki*; Kida, Tatsuya*; Watanabe, Wataru*; Nakajima, Yasutaka*; Fukuoka, Sachio*; Mori, Atsunori*; Matsumura, Tatsuro; Nakano, Yoshio*; Takeshita, Kenji*
Polymer Journal, 43(7), p.630 - 634, 2011/07
Times Cited Count:11 Percentile:35.53(Polymer Science) -(tetrakis-2-pyridylmethyl)ethylenediamine (TPEN) derivatives bearing a polymerizable double bond in the substituent structure of the pyridine ring are synthesized and subjected to copolymerization with
-(tetrakis-2-pyridylmethyl)ethylenediamine (TPEN) derivatives bearing a polymerizable double bond in the substituent structure of the pyridine ring are synthesized and subjected to copolymerization with  -isopropylacrylamide in the presence of AIBN. The obtained poly(TPEN-NIPA) gels show thermo-responsive swelling/shrinking behaviors and are employed for the extraction of cadmium(II) ion from the aqueous solution to examine the relationship of the gel characteristics and the extraction performance. The polymer gels composed of the TPEN derivative bearing C3, C4, C10 and branched C3 spacer chains are synthesized and temperature-dependent extraction behavior of cadmium ion is compared. These gels extract Cd(II) ion efficiently from the aqueous solution in the swelling state at 5
-isopropylacrylamide in the presence of AIBN. The obtained poly(TPEN-NIPA) gels show thermo-responsive swelling/shrinking behaviors and are employed for the extraction of cadmium(II) ion from the aqueous solution to examine the relationship of the gel characteristics and the extraction performance. The polymer gels composed of the TPEN derivative bearing C3, C4, C10 and branched C3 spacer chains are synthesized and temperature-dependent extraction behavior of cadmium ion is compared. These gels extract Cd(II) ion efficiently from the aqueous solution in the swelling state at 5 C, while little extraction is observed at 45
C, while little extraction is observed at 45 C with shrinking. It is found that poly(TPEN-NIPA) gel of branched C3 spacer (C3b) shows the excellent thermoresponsive extraction performance.
C with shrinking. It is found that poly(TPEN-NIPA) gel of branched C3 spacer (C3b) shows the excellent thermoresponsive extraction performance.
Matsumura, Tatsuro; Inaba, Yusuke*; Mori, Atsunori*; Takeshita, Kenji*
Journal of Nuclear Science and Technology, 47(2), p.123 - 126, 2010/02
Times Cited Count:12 Percentile:62.3(Nuclear Science & Technology)We are developing a new MA/Ln separation process with TPEN and its derivatives for P&T technology. TPEN is a hexadentate soft-donor ligand that has six soft-donor sites as a kind of podand type molecule and can encapsulate a metal ion. TPEN has good selectivity of Am(III) from Ln(III) and has potential to establish partitioning of MA. From the viewpoint of practical application, the hydrophobicity of TPEN must be improved. Because the dissolution of a slight amount of TPEN (about 10 mol/l) to water will restrict decontamination of Ln in extraction process. In this study, the hydrophobicity of TPEN was improved by introducing alkyl groups and the effect of the introduction of alkyl groups on the separation of Am(III) and Eu(III) was examined. One of them, N,N,N',N'-tetrakis((5-butoxypyridin-2-yl)-methyl)ethylenediamine, TBPEN, showed good selectivity and the maximum separation factor, SF
mol/l) to water will restrict decontamination of Ln in extraction process. In this study, the hydrophobicity of TPEN was improved by introducing alkyl groups and the effect of the introduction of alkyl groups on the separation of Am(III) and Eu(III) was examined. One of them, N,N,N',N'-tetrakis((5-butoxypyridin-2-yl)-methyl)ethylenediamine, TBPEN, showed good selectivity and the maximum separation factor, SF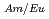 , was 91 at pH 3.02. The hydrophobicity of TBPEN is checked by the distribution data between water and chloroform, and improved successfully. The approach for development of hydrophobic derivatives of TPEN was effective.
, was 91 at pH 3.02. The hydrophobicity of TBPEN is checked by the distribution data between water and chloroform, and improved successfully. The approach for development of hydrophobic derivatives of TPEN was effective.
Fukuoka, Sachio*; Kida, Tatsuya*; Nakajima, Yasutaka*; Tsumagari, Takayuki*; Watanabe, Wataru*; Inaba, Yusuke*; Mori, Atsunori*; Matsumura, Tatsuro; Nakano, Yoshio*; Takeshita, Kenji*
Tetrahedron, 66(9), p.1721 - 1727, 2010/02
Times Cited Count:18 Percentile:48.53(Chemistry, Organic)N,N,N',N'-(tetrakis-2-pyridylmethyl)ethylenediamine (TPEN) derivatives bearing the different number (1-4) of a double bond moiety on the pyridine ring are synthesized and subjected to copolymerization with N-isopropylacrylamide in the presence of AIBN. The obtained poly(TPEN-NIPA)gels show thermo-responsive swelling/shrinking behaviors and are employed for the extraction of cadmium(II) ion from the aqueous solution to examine the relationship of the gel characteristics and the extraction performance. The polymer gels composed of the TPEN derivative bearing three or four double bonds exhibit temperature-dependent change of swelling and shrinking in water. These gels extract CdII ion efficiently from the aqueous solution in the swelling state at 5 C, while little extraction was observed at 45
C, while little extraction was observed at 45 C with shrinking.
C with shrinking.
Matsumura, Tatsuro; Takeshita, Kenji*; Inaba, Yusuke*; Mori, Atsunori*
Proceedings of 10th OECD/NEA Information Exchange Meeting on Actinide and Fission Product Partitioning and Transmutation (CD-ROM), 6 Pages, 2010/00
no abstracts in English
 -tetrakis((5-alkoxypyridin-2-yl)methyl)ethylenediamine, TRPEN, effective ligands for the separation of trivalent minor actinides from lanthanides
-tetrakis((5-alkoxypyridin-2-yl)methyl)ethylenediamine, TRPEN, effective ligands for the separation of trivalent minor actinides from lanthanidesMatsumura, Tatsuro; Inaba, Yusuke*; Koma, Yoshikazu; Morita, Yasuji; Mori, Atsunori*; Takeshita, Kenji*
Proceedings of International Conference on Advanced Nuclear Fuel Cycle; Sustainable Options & Industrial Perspectives (Global 2009) (CD-ROM), p.1107 - 1112, 2009/09
no abstracts in English
Matsumura, Tatsuro; Inaba, Yusuke*; Takeshita, Kenji*; Mori, Atsunori*
Proceedings of International Solvent Extraction Conference "Solvent Extraction-Fundamentals to Industrial Applications" (ISEC 2008), 4 Pages, 2008/09
We are developing a new MA/Ln separation process with derivatives of TPEN (N,N,N',N'-tetrakis(2-pyridylmethyl)ethylenediamine) for P&T technology. TPEN has good selectivity of Am(III) from Ln(III). However, TPEN is soluble slightly (about 10 mol/l) to water. In this study, the hydrophobicity of TPEN is improved by introducing alkyl groups and the selectivity of Am(III) and Eu(III) is examined. We succeeded to synthesize several new hydrophobic derivative of TPEN. One of the ligands, TDdPEN (N,N,N',N'-tetrakis((5-dodecyloxypyridin-2-yl)-methyl)ethylenediamine) showed high selectivity. The maximum separation factor, SF
mol/l) to water. In this study, the hydrophobicity of TPEN is improved by introducing alkyl groups and the selectivity of Am(III) and Eu(III) is examined. We succeeded to synthesize several new hydrophobic derivative of TPEN. One of the ligands, TDdPEN (N,N,N',N'-tetrakis((5-dodecyloxypyridin-2-yl)-methyl)ethylenediamine) showed high selectivity. The maximum separation factor, SF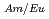 , was over 800. Hydrophobic derivatives of TPEN that have potential of application to the MA/Ln separation process was synthesized successfully.
, was over 800. Hydrophobic derivatives of TPEN that have potential of application to the MA/Ln separation process was synthesized successfully.
 -tetrakis(2-pyridylmethyl)ethylenediamine] derivatives bearing hydrophobic substituents
-tetrakis(2-pyridylmethyl)ethylenediamine] derivatives bearing hydrophobic substituentsInaba, Yusuke*; Nakajima, Yasutaka*; Fukuoka, Sachio*; Mori, Atsunori*; Takeshita, Kenji*; Matsumura, Tatsuro
no journal, ,
no abstracts in English
Fukuoka, Sachio*; Inaba, Yusuke*; Nakajima, Yasutaka*; Mori, Atsunori*; Takeshita, Kenji*; Matsumura, Tatsuro; Nakano, Yoshio*
no journal, ,
no abstracts in English
Matsumura, Tatsuro; Inaba, Yusuke*; Takeshita, Kenji*; Mori, Atsunori*
no journal, ,
no abstracts in English
Matsumura, Tatsuro; Inaba, Yusuke*; Takeshita, Kenji*; Mori, Atsunori*
no journal, ,
no abstracts in English
Matsumura, Tatsuro; Inaba, Yusuke*; Mori, Atsunori*; Takeshita, Kenji*
no journal, ,
no abstracts in English
Ogata, Takeshi*; Takeshita, Kenji*; Oaki, Hiroshi*; Inaba, Yusuke*; Mori, Atsunori*; Matsumura, Tatsuro; Nakano, Yoshio*
no journal, ,
N,N,N',N'-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) is one of the podand-type ligands with six nitrogen-donors and recognize the slight difference in the softness between Am(III) and Eu(III). We have tried to synthesize a novel extraction chromatographic agent, which exploit such feature of TPEN. The use of the polymer gel as a support of the extractant can be expect to suppress the elution of the extractant and keep the flexibility of extractant. N,N,N',N'-tetrakis-(4-propenyloxy-2-pyridylmethyl)ethylenediamine (TPPEN) which is TPEN analog introducing polymerizable group to pyridine ring was used as a cross-linker of N-isopropylacrylamide (NIPA). Furthermore, the TPPEN-NIPA gel on highly porous silica was prepared to improve the mechanical strength of the gel. These extraction chromatographic agents have been tested for the separation of Am(III) and Eu(III). Characterization and extraction performance of the extraction chromatographic agents will be described.
Inaba, Yusuke*; Kida, Tatsuya*; Watanabe, Wataru*; Mori, Atsunori*; Matsumura, Tatsuro; Ogata, Takeshi*; Takeshita, Kenji*
no journal, ,
Separation of minor actinides (MAs) from lanthanides in high-level radioactive waste (HLW) becomes important in nuclear power generation. Although TPEN is a possible candidate for the practical separating agent for MAs from HLW, there still remains difficulties in high water solubility and easy protonation characteristics. The practical MA separation is conducted from a mixture of MAs and Ln(III) in a highly acidic aqueous solution, thereby a hydrophobic and acid tolerant chelating agent needs to be developed. We herein report efficient syntheses of TPEN derivatives bearing various hydrophobic side-chains, which are alkyloxy and fluorinated alkoxy groups, and solvent extraction of Am(III) from Eu(III) in nitric acid aqueous solution. Synthesized TPEN derivatives were hydrophobic and showed higher performance than unsubstituted TPEN for Am separation from Eu in extraction with water/organic solvents at low pH region.
Suzuki, Shinichi; Akutsu, Kazuhiro; Yaita, Tsuyoshi; Okamoto, Yoshihiro; Ogata, Tsuyoshi*; Takeshita, Kenji*; Inaba, Yusuke*; Ikeda, Atsushi; Kobayashi, Toru; Oaki, Hiroshi*; et al.
no journal, ,
no abstracts in English