Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 /Md
/Md reduction potential studied with flow electrolytic chromatography
reduction potential studied with flow electrolytic chromatographyToyoshima, Atsushi; Li, Z.*; Asai, Masato; Sato, Nozomi; Sato, Tetsuya; Kikuchi, Takahiro; Kaneya, Yusuke; Kitatsuji, Yoshihiro; Tsukada, Kazuaki; Nagame, Yuichiro; et al.
Inorganic Chemistry, 52(21), p.12311 - 12313, 2013/11
Times Cited Count:6 Percentile:23.49(Chemistry, Inorganic & Nuclear)The reduction behavior of mendelevium (Md) was studied using a flow electrolytic chromatography apparatus. By applying appropriate potentials on the chromatography column, the more stable Md is reduced to Md
is reduced to Md . The reduction potential of the Md
. The reduction potential of the Md + e
+ e
 Md
Md couple was determined to be -0.16
couple was determined to be -0.16 0.05 V vs. a normal hydrogen electrode.
0.05 V vs. a normal hydrogen electrode.
 SO
SO /HNO
/HNO mixed solution
mixed solutionLi, Z.*; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Kikuchi, Takahiro; Nagame, Yuichiro; Sch del, M.; Pershina, V.*; et al.
del, M.; Pershina, V.*; et al.
Radiochimica Acta, 100(3), p.157 - 164, 2012/03
Times Cited Count:14 Percentile:68.93(Chemistry, Inorganic & Nuclear)Kasamatsu, Yoshitaka*; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Li, Z.; Ishii, Yasuo; Tome, Hayato*; Sato, Tetsuya; Kikuchi, Takahiro; Nishinaka, Ichiro; et al.
Chemistry Letters, 38(11), p.1084 - 1085, 2009/10
Times Cited Count:16 Percentile:48.84(Chemistry, Multidisciplinary)We report on the characteristic anion-exchange behavior of the superheavy element dubnium (Db) with atomic number Z = 105 in HF/HNO solution at the fluoride ion concentration [F
solution at the fluoride ion concentration [F ] = 0.003 M. The result clearly demonstrates that the fluoro complex formation of Db is significantly different from that of the group-5 homologue Ta in the 6th period of the periodic table while the behavior of Db is similar to that of the lighter homologue Nb in the 5th period.
] = 0.003 M. The result clearly demonstrates that the fluoro complex formation of Db is significantly different from that of the group-5 homologue Ta in the 6th period of the periodic table while the behavior of Db is similar to that of the lighter homologue Nb in the 5th period.
Kasamatsu, Yoshitaka; Toyoshima, Atsushi; Tsukada, Kazuaki; Asai, Masato; Ishii, Yasuo; Nishinaka, Ichiro; Sato, Tetsuya; Li, Z.; Kikuchi, Takahiro; Nagame, Yuichiro; et al.
no journal, ,
Radioactive nuclides of Nb and Ta were produced in the Zr/Hf(p,xn)Nb/Ta and Ge/Gd( F,xn)Nb/Ta reactions at the JAEA tandem accelerator. The products were rapidly transported by He/KCl and He/KF gas-jet systems to the chemistry laboratory. It was found that the transport efficiency using the He/KF gas-jet system is comparable with that by the He/KCl gas-jet system which has been generally used. Subsequently, on-line anion-exchange experiments with the products were performed in HF/HNO
F,xn)Nb/Ta reactions at the JAEA tandem accelerator. The products were rapidly transported by He/KCl and He/KF gas-jet systems to the chemistry laboratory. It was found that the transport efficiency using the He/KF gas-jet system is comparable with that by the He/KCl gas-jet system which has been generally used. Subsequently, on-line anion-exchange experiments with the products were performed in HF/HNO solutions. A small physical adsorption of Nb on the surfaces of the tools was observed when the KCl aerosols were used, while better reproducibility of the elution of Nb was obtained using the KF aerosols. We interpret that stable fluoro complexes of Nb are rapidly formed by using the KF aerosols and we can suggest that the He/KF gas-jet system should be used in the on-line anion-exchange experiment of the group 5 elements for the study of the fluoride complexation of the elements.
solutions. A small physical adsorption of Nb on the surfaces of the tools was observed when the KCl aerosols were used, while better reproducibility of the elution of Nb was obtained using the KF aerosols. We interpret that stable fluoro complexes of Nb are rapidly formed by using the KF aerosols and we can suggest that the He/KF gas-jet system should be used in the on-line anion-exchange experiment of the group 5 elements for the study of the fluoride complexation of the elements.
 solutions using a newly developed on-line experimental system
solutions using a newly developed on-line experimental systemTsukada, Kazuaki; Kasamatsu, Yoshitaka; Asai, Masato; Toyoshima, Atsushi; Ishii, Yasuo; Li, Z.; Kikuchi, Takahiro; Sato, Tetsuya; Nishinaka, Ichiro; Nagame, Yuichiro; et al.
no journal, ,
Anion-exchange chromatographic behavior of element 105, dubnium (Db), produced in the  Cm(
Cm( F,5n)
F,5n) Db reaction is investigated together with the homologues Nb and Ta in HF/HNO
Db reaction is investigated together with the homologues Nb and Ta in HF/HNO mixed media using a newly developed on-line experimental system. The result indicates that the adsorption sequence on the anion-exchange resin is Ta
mixed media using a newly developed on-line experimental system. The result indicates that the adsorption sequence on the anion-exchange resin is Ta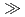 Nb
Nb Db, and the fluoride complex formation of Db is expected to be weaker than that of homologues.
Db, and the fluoride complex formation of Db is expected to be weaker than that of homologues.
 mixed solution using a new on-line chemical apparatus
mixed solution using a new on-line chemical apparatusTsukada, Kazuaki; Kasamatsu, Yoshitaka*; Asai, Masato; Toyoshima, Atsushi; Ishii, Yasuo; Li, Z.; Kikuchi, Takahiro; Sato, Tetsuya; Nishinaka, Ichiro; Nagame, Yuichiro; et al.
no journal, ,
Anion-exchange chromatographic behavior of element 105, dubnium (Db), produced in the  Cm(
Cm( F,5n) reaction is investigated together with the homologues Nb and Ta in HF/HNO
F,5n) reaction is investigated together with the homologues Nb and Ta in HF/HNO mixed media using a newly developed on-line experimental system. The result indicates that the adsorption sequence on the anion-exchange resin is Ta
mixed media using a newly developed on-line experimental system. The result indicates that the adsorption sequence on the anion-exchange resin is Ta 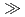 Nb
Nb  Db, and the fluoride complex formation of Db is expected to be weaker than that of homologues.
Db, and the fluoride complex formation of Db is expected to be weaker than that of homologues.
 SO
SO /HNO
/HNO mixed solutions; Towards to study on sulfate complexation of
mixed solutions; Towards to study on sulfate complexation of 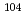 Rf
RfLi, Z.; Toyoshima, Atsushi; Tsukada, Kazuaki; Asai, Masato; Sato, Tetsuya; Kikuchi, Takahiro; Sato, Nozomi; Nagame, Yuichiro
no journal, ,
Toyoshima, Atsushi; Tsukada, Kazuaki; Asai, Masato; Sato, Tetsuya; Li, Z.; Sato, Nozomi; Kikuchi, Takahiro; Kitatsuji, Yoshihiro; Nagame, Yuichiro; Oe, Kazuhiro*; et al.
no journal, ,
Reduction of element 101, mendelevium (Md), was studied with flow electrolytic chromatography. Mendelevium-255 with a half-life of 27 min was produced in the  Cm(
Cm(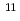 B, 4n) reaction at the JAEA tandem accelerator. Reaction products transported by a KCl/He gas-jet method to the chemistry laboratory were collected on a chemistry apparatus. After removing KCl with HDEHP chromatography, elution behavior of Md in 0.1 M HCl was investigated with a flow electrolytic column apparatus at the applied potentials between 0
B, 4n) reaction at the JAEA tandem accelerator. Reaction products transported by a KCl/He gas-jet method to the chemistry laboratory were collected on a chemistry apparatus. After removing KCl with HDEHP chromatography, elution behavior of Md in 0.1 M HCl was investigated with a flow electrolytic column apparatus at the applied potentials between 0  -0.9 V vs. a Ag/AgCl reference electrode. Elution behavior of Md was the same as that of
-0.9 V vs. a Ag/AgCl reference electrode. Elution behavior of Md was the same as that of 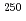 Bk
Bk at 0 V, showing that the stable Md
at 0 V, showing that the stable Md was not reduced to the divalent state. At -0.9 V, elution of Md was quite similar to that of Sr
was not reduced to the divalent state. At -0.9 V, elution of Md was quite similar to that of Sr , demonstrating that Md
, demonstrating that Md was successfully reduced to Md
was successfully reduced to Md .
.
Toyoshima, Atsushi; Tsukada, Kazuaki; Asai, Masato; Kitatsuji, Yoshihiro; Ishii, Yasuo; Sato, Tetsuya; Li, Z.; Sato, Nozomi; Kikuchi, Takahiro; Nishinaka, Ichiro; et al.
no journal, ,
Oxidation of element 102, nobelium (No), using a developed electrolytic chromatography apparatus on an atom-at-a-time scale will be presented. It was found that the most stable ion, No , is oxidized to No
, is oxidized to No in
in  -hydroxyisobutyric acid (
-hydroxyisobutyric acid ( -HIB) solution using the apparatus and that the oxidized No complex with
-HIB) solution using the apparatus and that the oxidized No complex with  -HIB holds the trivalent state in the column above an applied potential of 1.0 V. Electrochemical reduction of element 101, mendevelium (Md) in HCl will be also presented.
-HIB holds the trivalent state in the column above an applied potential of 1.0 V. Electrochemical reduction of element 101, mendevelium (Md) in HCl will be also presented.
Sato, Tetsuya; Tsukada, Kazuaki; Asai, Masato; Toyoshima, Atsushi; Kasamatsu, Yoshitaka*; Li, Z.; Sato, Nozomi; Kikuchi, Takahiro; Nagame, Yuichiro
no journal, ,
To investigate chemical properties of the transactinide element dubnium (Db, Z=105), we have developed an on-line isothermal gas chromatographic apparatus. The on-line experiments with the group-5 elements using the short lived  Nb and
Nb and 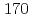 Ta as the homologues of Db were conducted. We measured the relative chemical yields of volatile compounds of
Ta as the homologues of Db were conducted. We measured the relative chemical yields of volatile compounds of 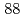 Nb and
Nb and 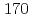 Ta as a function of the isothermal temperature in chlorinating condition with mixture of air and the SOCl
Ta as a function of the isothermal temperature in chlorinating condition with mixture of air and the SOCl vapor. It was found that the volatility of compounds of Ta is lower than that of Nb.
vapor. It was found that the volatility of compounds of Ta is lower than that of Nb.
Sato, Tetsuya; Tsukada, Kazuaki; Asai, Masato; Toyoshima, Atsushi; Kasamatsu, Yoshitaka*; Li, Z.; Sato, Nozomi; Kikuchi, Takahiro; Haba, Hiromitsu*; Goto, Shinichi*; et al.
no journal, ,
To investigate chemical properties of the transactinide elements dubnium (Db, Z = 105) and seaborgium (Sg, Z = 106), we have developed an on-line isothermal gas chromatographic apparatus. The on-line experiments with the group-5 and -6 elements using the short lived 88Nb and 170Ta as the homologues of Db and 173W as the one of Sg were conducted. We measured the relative chemical yields of volatile compounds of 88Nb, 170Ta and 173W as a function of the isothermal temperature. The behavior of W and Nb was the same as that obtained in previous work by M. Gartner et al., and A. Turler et al., respectively. It was found that the volatility of compounds of Ta is lower than that of Nb.
Nagame, Yuichiro; Asai, Masato; Ishii, Yasuo; Kikuchi, Takahiro; Li, Z.; Sato, Tetsuya; Toyoshima, Atsushi; Tsukada, Kazuaki; Kasamatsu, Yoshitaka*; Haba, Hiromitsu*; et al.
no journal, ,
no abstracts in English
 -HiB complexation of Zr and Hf as homologues of Rf
-HiB complexation of Zr and Hf as homologues of RfKikuchi, Takahiro; Toyoshima, Atsushi; Li, Z.; Tsukada, Kazuaki; Asai, Masato; Sato, Tetsuya; Sato, Nozomi; Nagame, Yuichiro; Kasamatsu, Yoshitaka*; Fan, F.*
no journal, ,
The ion-exchange behavior of Zr and Hf in  -HiB / HNO
-HiB / HNO mixed solutions has been studied by a batch method using the carrier-free radiotracers
mixed solutions has been studied by a batch method using the carrier-free radiotracers  Zr and
Zr and 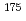 Hf. It is found that the
Hf. It is found that the  -HiB complex formation of Zr and Hf reaches equilibrium in 180 min and that the ion-exchange behavior of these elements are basically similar to each other. The distribution coefficients of Zr and Hf on the cation-exchange resin at [H
-HiB complex formation of Zr and Hf reaches equilibrium in 180 min and that the ion-exchange behavior of these elements are basically similar to each other. The distribution coefficients of Zr and Hf on the cation-exchange resin at [H ]
]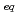 = 0.1 M decrease with an increase of [
= 0.1 M decrease with an increase of [ -HiB
-HiB ]
]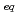 , reflecting successive formation of
, reflecting successive formation of  -HiB complexes.
-HiB complexes.
 mixed solution
mixed solutionKasamatsu, Yoshitaka*; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Li, Z.; Ishii, Yasuo; Sato, Tetsuya; Nishinaka, Ichiro; Kikuchi, Takahiro; Haba, Hiromitsu*; et al.
no journal, ,
Dubnium-262 was produced in the  Cm(
Cm( F, 5n) reaction at the JAEA tandem accelerator. Reaction products were rapidly transported by a He/KF gas-jet system to the chemistry laboratory. Anion-exchange behavior of Db in HF/HNO
F, 5n) reaction at the JAEA tandem accelerator. Reaction products were rapidly transported by a He/KF gas-jet system to the chemistry laboratory. Anion-exchange behavior of Db in HF/HNO solution was then investigated using the newly developed on-line ion-exchange and
solution was then investigated using the newly developed on-line ion-exchange and  -particle detection apparatus. Distribution coefficients of Db were clearly smaller than those of Ta, the closest homologue in the periodic table, and were close to those of lighter homologue Nb and also the pseudo homologue Pa. Fluoride complex formation of Db will be discussed.
-particle detection apparatus. Distribution coefficients of Db were clearly smaller than those of Ta, the closest homologue in the periodic table, and were close to those of lighter homologue Nb and also the pseudo homologue Pa. Fluoride complex formation of Db will be discussed.
Toyoshima, Atsushi; Tsukada, Kazuaki; Asai, Masato; Sato, Tetsuya; Li, Z.; Sato, Nozomi; Kikuchi, Takahiro; Kitatsuji, Yoshihiro; Nagame, Yuichiro; Oe, Kazuhiro*; et al.
no journal, ,
Reduction of mendelevium (Md) was studied by an electrochemical method on a single atom scale. Mendelevium-255 produced in the  Cm(
Cm(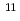 B,4n) reaction at the JAEA tandem accelerator was transported to a chemistry laboratory by a gas-jet method. The Md isotope was then dissolved with 0.1 M HCl and fed into a working electrode modified with cation-exchange resin of a flow electrolytic column apparatus. The elution behavior on the electrode was examined as a function of the applied potential. It was found that trivalent ion of Md is reduced to the divalent state at the potentials below -0.4 V vs. an Ag/AgCl reference electrode.
B,4n) reaction at the JAEA tandem accelerator was transported to a chemistry laboratory by a gas-jet method. The Md isotope was then dissolved with 0.1 M HCl and fed into a working electrode modified with cation-exchange resin of a flow electrolytic column apparatus. The elution behavior on the electrode was examined as a function of the applied potential. It was found that trivalent ion of Md is reduced to the divalent state at the potentials below -0.4 V vs. an Ag/AgCl reference electrode.
Toyoshima, Atsushi; Tsukada, Kazuaki; Asai, Masato; Kitatsuji, Yoshihiro; Ishii, Yasuo; Sato, Tetsuya; Li, Z.; Sato, Nozomi; Kikuchi, Takahiro; Nishinaka, Ichiro; et al.
no journal, ,
Electrochemical studies of nobelium (No) and mendelevium (Md) using an electrolytic column chromatography at JAEA will be presented. The isotopes  No and
No and  Md were produced in the
Md were produced in the  Cm(
Cm( C, 5n) and
C, 5n) and  Cm(
Cm(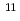 B, 4n) reactions, respectively, at the JAEA tandem accelerator. In the experiment of No, the oxidation of No
B, 4n) reactions, respectively, at the JAEA tandem accelerator. In the experiment of No, the oxidation of No to No
to No in 0.1 M
in 0.1 M  -hydroxyisobutyric acid (
-hydroxyisobutyric acid ( -HIB) was studied with comparing with the elution of Sr
-HIB) was studied with comparing with the elution of Sr and Yb
and Yb . At the applied potential of 0.2 V, No was not eluted with
. At the applied potential of 0.2 V, No was not eluted with  -HIB. This adsorption of No was the same as that of Sr
-HIB. This adsorption of No was the same as that of Sr , indicating that No was bound in its stable divalent state. On the other hand, the elution of No in
, indicating that No was bound in its stable divalent state. On the other hand, the elution of No in  -HIB was observed at 1.2 V at the position of Yb
-HIB was observed at 1.2 V at the position of Yb , which demonstrated that No
, which demonstrated that No was successfully oxidized to No
was successfully oxidized to No . Reduction of Md
. Reduction of Md to Md
to Md was also successfully carried out with the apparatus in 0.1 M HCl.
was also successfully carried out with the apparatus in 0.1 M HCl.
 mixed solution using an on-line chemical apparatus
mixed solution using an on-line chemical apparatusTsukada, Kazuaki; Kasamatsu, Yoshitaka*; Asai, Masato; Toyoshima, Atsushi; Ishii, Yasuo; Li, Z.; Kikuchi, Takahiro; Sato, Tetsuya; Nagame, Yuichiro; Sch del, M.; et al.
del, M.; et al.
no journal, ,
We have investigated the chemical behavior of Db together with its group-5 homologues by anion-exchange chromatography in HF/HNO mixed solution using a rapid online chemical apparatus (AIDA-II). The nuclides
mixed solution using a rapid online chemical apparatus (AIDA-II). The nuclides  Db,
Db,  Nb and
Nb and 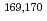 Ta were produced in the
Ta were produced in the  Cm(
Cm( F,
F, 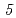 n),
n),  Ge(
Ge( F,
F,  n) and
n) and 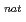 Gd(
Gd( F,
F,  n) reactions, respectively, at the JAEA tandem accelerator. On-line anion-exchange separations of Db, Nb and Ta were performed using the AIDA-II. Thousand times of anion-exchange separations were conducted using AIDA-II.
n) reactions, respectively, at the JAEA tandem accelerator. On-line anion-exchange separations of Db, Nb and Ta were performed using the AIDA-II. Thousand times of anion-exchange separations were conducted using AIDA-II.  events were registered, and the
events were registered, and the 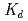 values for Db was evaluated. From these results, the adsorption sequence Pa
values for Db was evaluated. From these results, the adsorption sequence Pa  Db
Db  Nb
Nb 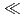 Ta was determined. The present result shows a notable difference in the adsorption behavior between Db and its homologue Ta. In the conference, the present status and the perspective of the aqueous chemistry of Db at JAEA will be also presented.
Ta was determined. The present result shows a notable difference in the adsorption behavior between Db and its homologue Ta. In the conference, the present status and the perspective of the aqueous chemistry of Db at JAEA will be also presented.
 SO
SO /HNO
/HNO mixed solution ([H
mixed solution ([H ] = 1.0 M)
] = 1.0 M)Li, Z.; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Kikuchi, Takahiro; Sch del, M.*; Nagame, Yuichiro; Liang, X. H.*; et al.
del, M.*; Nagame, Yuichiro; Liang, X. H.*; et al.
no journal, ,
Cation-exchange behavior of  Rf (T
Rf (T = 78 s) produced in the
= 78 s) produced in the  Cm(
Cm( O, 5n) reaction was studied on a "one-atom-at-a-time" scale in H
O, 5n) reaction was studied on a "one-atom-at-a-time" scale in H SO
SO (0.15-0.69 M)/HNO
(0.15-0.69 M)/HNO mixed solutions ([H
mixed solutions ([H ] = 1.0 M) using an automated ion-exchange separation apparatus coupled with the detection system for alpha-spectroscopy. It was found that adsorption probability (%ads) of
] = 1.0 M) using an automated ion-exchange separation apparatus coupled with the detection system for alpha-spectroscopy. It was found that adsorption probability (%ads) of  Rf on cation-exchange resin decreases with increasing [HSO
Rf on cation-exchange resin decreases with increasing [HSO
 ], showing a successive formation of its sulfate complexes. Rf exhibited much weaker formation of the complexes than the lighter homologues Zr and Hf, which is qualitatively in good agreement with theoretical calculations including relativistic effects.
], showing a successive formation of its sulfate complexes. Rf exhibited much weaker formation of the complexes than the lighter homologues Zr and Hf, which is qualitatively in good agreement with theoretical calculations including relativistic effects.
 -HiB complexation and ion-exchange behavior of the group-4 elements as homologues of Rf
-HiB complexation and ion-exchange behavior of the group-4 elements as homologues of RfKikuchi, Takahiro; Toyoshima, Atsushi; Li, Z.; Tsukada, Kazuaki; Asai, Masato; Sato, Tetsuya; Sato, Nozomi; Nagame, Yuichiro; Kasamatsu, Yoshitaka*; Fan, F.*
no journal, ,
We studied complexation of the group-4 elements Zr and Hf as well as the pseudo group-4 element Th with  -hydroxyisobutyric acid (
-hydroxyisobutyric acid ( -HiB) as homologues of Element 104, rutherfordium (Rf). The isotopes of
-HiB) as homologues of Element 104, rutherfordium (Rf). The isotopes of  Zr and
Zr and 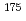 Hf were produced at the JAEA tandem accelerator while
Hf were produced at the JAEA tandem accelerator while  Th was separated from
Th was separated from  U. These radiotracers stocked in
U. These radiotracers stocked in  -HiB/HNO
-HiB/HNO were mixed with cation-exchange resin. After shaking the sample for a certain time, an aliquot of the aqueous phase was precisely taken, which were subjected to
were mixed with cation-exchange resin. After shaking the sample for a certain time, an aliquot of the aqueous phase was precisely taken, which were subjected to  -spectrometry. The complex formation of Zr and Hf reached to chemical equlibria at 180 min. Distribution coefficients of Zr and Hf were decreased with an increase of the concentration of
-spectrometry. The complex formation of Zr and Hf reached to chemical equlibria at 180 min. Distribution coefficients of Zr and Hf were decreased with an increase of the concentration of  -HiB, indicating the consecutive complexation of these ions with
-HiB, indicating the consecutive complexation of these ions with  -HiB. On the other hand, Th was adsorbed on the cation-exchange resin under the given conditions, showing clearly different behavior of Th from that of Zr and Hf.
-HiB. On the other hand, Th was adsorbed on the cation-exchange resin under the given conditions, showing clearly different behavior of Th from that of Zr and Hf.
 mixed solutions; Towards the study of ion-exchange behavior of
mixed solutions; Towards the study of ion-exchange behavior of 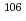 Sg
SgLiang, X.; Li, Z.; Tsukada, Kazuaki; Toyoshima, Atsushi; Asai, Masato; Sato, Tetsuya; Sato, Nozomi; Kikuchi, Takahiro; Kaneya, Yusuke; Nagame, Yuichiro
no journal, ,
Anion-exchange behavior of the group-6 elements, W and Mo, in HF and HNO mixed solutions has been studied by a batch method using the carrier-free radiotracers
mixed solutions has been studied by a batch method using the carrier-free radiotracers 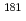 W and
W and 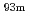 Mo. The long-lived isotope
Mo. The long-lived isotope  W and the short-lived isotope
W and the short-lived isotope 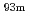 Mo were produced in the
Mo were produced in the  Ta(p,n) and the
Ta(p,n) and the 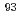 Nb(p,n) reaction at the JAEA tandem accelerator, respectively. The solutions containing
Nb(p,n) reaction at the JAEA tandem accelerator, respectively. The solutions containing 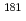 W or
W or 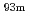 Mo were mixed with anion exchange resin. Radioactivities of the solution were assayed for
Mo were mixed with anion exchange resin. Radioactivities of the solution were assayed for  -ray spectrometry, and then distribution coefficients (K
-ray spectrometry, and then distribution coefficients (K s) of these elements were determined. It was found that the K
s) of these elements were determined. It was found that the K values of W depend strongly on the concentration of HF, while those of Mo are almost constant as a function of [HF] under the given conditions.
values of W depend strongly on the concentration of HF, while those of Mo are almost constant as a function of [HF] under the given conditions.