Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kasamatsu, Yoshitaka*; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Li, Z.; Ishii, Yasuo; Tome, Hayato*; Sato, Tetsuya; Kikuchi, Takahiro; Nishinaka, Ichiro; et al.
Chemistry Letters, 38(11), p.1084 - 1085, 2009/10
Times Cited Count:16 Percentile:48.84(Chemistry, Multidisciplinary)We report on the characteristic anion-exchange behavior of the superheavy element dubnium (Db) with atomic number Z = 105 in HF/HNO solution at the fluoride ion concentration [F
solution at the fluoride ion concentration [F ] = 0.003 M. The result clearly demonstrates that the fluoro complex formation of Db is significantly different from that of the group-5 homologue Ta in the 6th period of the periodic table while the behavior of Db is similar to that of the lighter homologue Nb in the 5th period.
] = 0.003 M. The result clearly demonstrates that the fluoro complex formation of Db is significantly different from that of the group-5 homologue Ta in the 6th period of the periodic table while the behavior of Db is similar to that of the lighter homologue Nb in the 5th period.
 solutions using a newly developed on-line experimental system
solutions using a newly developed on-line experimental systemTsukada, Kazuaki; Kasamatsu, Yoshitaka; Asai, Masato; Toyoshima, Atsushi; Ishii, Yasuo; Li, Z.; Kikuchi, Takahiro; Sato, Tetsuya; Nishinaka, Ichiro; Nagame, Yuichiro; et al.
no journal, ,
Anion-exchange chromatographic behavior of element 105, dubnium (Db), produced in the  Cm(
Cm( F,5n)
F,5n) Db reaction is investigated together with the homologues Nb and Ta in HF/HNO
Db reaction is investigated together with the homologues Nb and Ta in HF/HNO mixed media using a newly developed on-line experimental system. The result indicates that the adsorption sequence on the anion-exchange resin is Ta
mixed media using a newly developed on-line experimental system. The result indicates that the adsorption sequence on the anion-exchange resin is Ta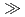 Nb
Nb Db, and the fluoride complex formation of Db is expected to be weaker than that of homologues.
Db, and the fluoride complex formation of Db is expected to be weaker than that of homologues.
 mixed solution using a new on-line chemical apparatus
mixed solution using a new on-line chemical apparatusTsukada, Kazuaki; Kasamatsu, Yoshitaka*; Asai, Masato; Toyoshima, Atsushi; Ishii, Yasuo; Li, Z.; Kikuchi, Takahiro; Sato, Tetsuya; Nishinaka, Ichiro; Nagame, Yuichiro; et al.
no journal, ,
Anion-exchange chromatographic behavior of element 105, dubnium (Db), produced in the  Cm(
Cm( F,5n) reaction is investigated together with the homologues Nb and Ta in HF/HNO
F,5n) reaction is investigated together with the homologues Nb and Ta in HF/HNO mixed media using a newly developed on-line experimental system. The result indicates that the adsorption sequence on the anion-exchange resin is Ta
mixed media using a newly developed on-line experimental system. The result indicates that the adsorption sequence on the anion-exchange resin is Ta 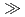 Nb
Nb  Db, and the fluoride complex formation of Db is expected to be weaker than that of homologues.
Db, and the fluoride complex formation of Db is expected to be weaker than that of homologues.
 mixed solution
mixed solutionKasamatsu, Yoshitaka*; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Li, Z.; Ishii, Yasuo; Sato, Tetsuya; Nishinaka, Ichiro; Kikuchi, Takahiro; Haba, Hiromitsu*; et al.
no journal, ,
Dubnium-262 was produced in the  Cm(
Cm( F, 5n) reaction at the JAEA tandem accelerator. Reaction products were rapidly transported by a He/KF gas-jet system to the chemistry laboratory. Anion-exchange behavior of Db in HF/HNO
F, 5n) reaction at the JAEA tandem accelerator. Reaction products were rapidly transported by a He/KF gas-jet system to the chemistry laboratory. Anion-exchange behavior of Db in HF/HNO solution was then investigated using the newly developed on-line ion-exchange and
solution was then investigated using the newly developed on-line ion-exchange and  -particle detection apparatus. Distribution coefficients of Db were clearly smaller than those of Ta, the closest homologue in the periodic table, and were close to those of lighter homologue Nb and also the pseudo homologue Pa. Fluoride complex formation of Db will be discussed.
-particle detection apparatus. Distribution coefficients of Db were clearly smaller than those of Ta, the closest homologue in the periodic table, and were close to those of lighter homologue Nb and also the pseudo homologue Pa. Fluoride complex formation of Db will be discussed.
 mixed solution using an on-line chemical apparatus
mixed solution using an on-line chemical apparatusTsukada, Kazuaki; Kasamatsu, Yoshitaka*; Asai, Masato; Toyoshima, Atsushi; Ishii, Yasuo; Li, Z.; Kikuchi, Takahiro; Sato, Tetsuya; Nagame, Yuichiro; Sch del, M.; et al.
del, M.; et al.
no journal, ,
We have investigated the chemical behavior of Db together with its group-5 homologues by anion-exchange chromatography in HF/HNO mixed solution using a rapid online chemical apparatus (AIDA-II). The nuclides
mixed solution using a rapid online chemical apparatus (AIDA-II). The nuclides  Db,
Db,  Nb and
Nb and 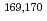 Ta were produced in the
Ta were produced in the  Cm(
Cm( F,
F, 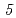 n),
n), 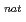 Ge(
Ge( F,
F,  n) and
n) and 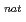 Gd(
Gd( F,
F,  n) reactions, respectively, at the JAEA tandem accelerator. On-line anion-exchange separations of Db, Nb and Ta were performed using the AIDA-II. Thousand times of anion-exchange separations were conducted using AIDA-II.
n) reactions, respectively, at the JAEA tandem accelerator. On-line anion-exchange separations of Db, Nb and Ta were performed using the AIDA-II. Thousand times of anion-exchange separations were conducted using AIDA-II.  events were registered, and the
events were registered, and the 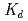 values for Db was evaluated. From these results, the adsorption sequence Pa
values for Db was evaluated. From these results, the adsorption sequence Pa  Db
Db  Nb
Nb 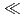 Ta was determined. The present result shows a notable difference in the adsorption behavior between Db and its homologue Ta. In the conference, the present status and the perspective of the aqueous chemistry of Db at JAEA will be also presented.
Ta was determined. The present result shows a notable difference in the adsorption behavior between Db and its homologue Ta. In the conference, the present status and the perspective of the aqueous chemistry of Db at JAEA will be also presented.
Takayama, Reona*; Oe, Kazuhiro*; Komori, Yukiko*; Fujisawa, Hiroyuki*; Kuriyama, Ai*; Kikutani, Yuki*; Kikunaga, Hidetoshi*; Kasamatsu, Yoshitaka*; Yoshimura, Takashi*; Takahashi, Naruto*; et al.
no journal, ,
The extraction behavior of trivalent actinides (Am, Cm, Cf, Es, and Fm) into di(2-ethylhexyl)phosphoric acid, HDEHP, in benzene from HNO was studied together with lanthanides. The extraction constants,
was studied together with lanthanides. The extraction constants, 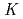
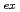 values, of lanthanides, Am, Cm, Cf, and Es were determined by a batch method using stable lanthanide isotopes and radiotracers of
values, of lanthanides, Am, Cm, Cf, and Es were determined by a batch method using stable lanthanide isotopes and radiotracers of  Am,
Am,  Cm,
Cm,  Cf, and
Cf, and 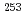 Es, respectively, while that of Fm was measured with
Es, respectively, while that of Fm was measured with 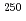 Fm which was produced in the
Fm which was produced in the  U(
U(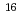 O,4n) reaction and was on-line supplied by a gas-jet method at the AVF cyclotron of RCNP, Osaka University. The
O,4n) reaction and was on-line supplied by a gas-jet method at the AVF cyclotron of RCNP, Osaka University. The 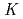
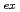 values were determined by the slope analysis for the variation of the distribution ratio as a function of the concentration of HDEHP. We found that the
values were determined by the slope analysis for the variation of the distribution ratio as a function of the concentration of HDEHP. We found that the 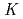
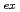 of Fm is smaller than that of Dy in spite of the similar ionic radii while those of other actinides are almost the same as those of each lanthanide with a similar ionic radius.
of Fm is smaller than that of Dy in spite of the similar ionic radii while those of other actinides are almost the same as those of each lanthanide with a similar ionic radius.