Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Miyahara, Shinya; ; Watanabe, Tomoo; Haga, Kazuo; Himeno, Yoshiaki
Nuclear Technology, 97(2), p.177 - 185, 1992/02
Times Cited Count:7 Percentile:57.42(Nuclear Science & Technology)None
Haga, Kazuo; ; Watanabe, Tomoo; ; Himeno, Yoshiaki
PNC TN9410 91-091, 13 Pages, 1991/01
A series of tests has been conducted to obtain gas-liquid equilibrium partition coefficient Kd of volatile fission products such as cesium,iodine,and tellurium in sodium. In the test a sodium pool mixed with an FP simulant was heated by an electric furnace and the solvent of trapped vapors by filters was quantitatively analyzed. The results are,(1)Cs shows the highest Kd (20-100), (2)Kd of iodine scatters as wide as 0.02-0.5at 450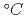 and 0.3-0.8 at 650
and 0.3-0.8 at 650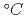 ,(3)the Kd values of Cs and I agree well with the theoretical ones reported by Castleman et al., and (4)if a sodium-telluride which is hard to vaporize than pure Te is assumed, measured Kd of Te agrees with that theoretical.
,(3)the Kd values of Cs and I agree well with the theoretical ones reported by Castleman et al., and (4)if a sodium-telluride which is hard to vaporize than pure Te is assumed, measured Kd of Te agrees with that theoretical.
 C-800
C-800 C
C*; *; *; *; *
PNC TN9410 89-034, 56 Pages, 1989/02
The saturated solubility of NaI in sodium was measured to be used for the safety analysis of the Fast Breeder Reactors. A saturated preparation vessel, and test apparatus was designed and preparation procedures for the study was established. The test was conducted in the sodium temperature range of 500 C-800
C-800 C. From the resu1ts obtained the two empirical equations saturated solubility have been derived: [log
C. From the resu1ts obtained the two empirical equations saturated solubility have been derived: [log S
S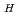 = 8.557-3524/T(800
= 8.557-3524/T(800 C-661.4
C-661.4 C)] [log
C)] [log S
S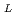 = 9.751-4640/T(661.4
= 9.751-4640/T(661.4 C-500
C-500 C)] [S
C)] [S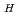 , S
, S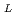 : saturated solubility (ppm)] [T : absolute temperature (K)] The above two equations of the saturated solubility represented test result with 1% errors. An intersection of the equations completely agreed with the melting point 661.4
: saturated solubility (ppm)] [T : absolute temperature (K)] The above two equations of the saturated solubility represented test result with 1% errors. An intersection of the equations completely agreed with the melting point 661.4 C of NaI. The results with the present ones by M, A, Bredig et al. agrees.
C of NaI. The results with the present ones by M, A, Bredig et al. agrees.