Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Okamoto, Yoshihiro; Osugi, Takeshi; Shiwaku, Hideaki; Akabori, Mitsuo*
Insights Concerning the Fukushima Daiichi Nuclear Accident, Vol.4; Endeavors by Scientists, p.285 - 294, 2021/10
The transfer behavior of cesium adsorbed on some clay minerals in aqueous solution was investigated by X-ray absorption fine structure (XAFS) analysis of the Cs K-edge. The sample was prepared by mixing Cs-adsorbed mineral with a different pure clay mineral in water. The XAFS results of the dried mixture powder were compared with those obtained before mixing. It was recognized from the XAFS analysis for three kinds of clay minerals illite, kaolinite and vermiculite, that cesium was transferred from kaolinite to illite and vermiculite, and from illite to vermiculite. It can be concluded that cesium is transferred to and accumulated in vermiculite.
Okamoto, Yoshihiro; Osugi, Takeshi; Akabori, Mitsuo; Kobayashi, Toru; Shiwaku, Hideaki
Journal of Molecular Liquids, 232, p.285 - 289, 2017/04
Times Cited Count:3 Percentile:12.21(Chemistry, Physical)High energy XAFS measurement using cerium K-edge was performed to study the chemical state of cerium in high-temperature molten slag (SiO -CaO-Fe
-CaO-Fe O
O -CeO
-CeO ). It was found from the change in the nearest Ce-O distance obtained from EXAFS analysis and the energetic shift of the white line peak observed in XANES analysis that oxidation state of cerium was tetravalent in the molten state and trivalent in solid state. The Debye-Waller factor of the nearest Ce-O pair in solid slag was very large even at room temperature, and the change in its value upon heating and melting was very small. This result suggests that cerium is highly disordered and stable in solid slag.
). It was found from the change in the nearest Ce-O distance obtained from EXAFS analysis and the energetic shift of the white line peak observed in XANES analysis that oxidation state of cerium was tetravalent in the molten state and trivalent in solid state. The Debye-Waller factor of the nearest Ce-O pair in solid slag was very large even at room temperature, and the change in its value upon heating and melting was very small. This result suggests that cerium is highly disordered and stable in solid slag.
Okamoto, Yoshihiro; Shiwaku, Hideaki; Nakada, Masami; Komamine, Satoshi*; Ochi, Eiji*; Akabori, Mitsuo
Journal of Nuclear Materials, 471, p.110 - 115, 2016/02
Times Cited Count:6 Percentile:49.29(Materials Science, Multidisciplinary)Extended X-ray Absorption Fine Structure (EXAFS) analyses were performed to evaluate REDOX (REDuction and OXidation) state of platinoid elements in simulated high-level nuclear waste glass samples prepared under different conditions of temperature and atmosphere. At first, EXAFS functions were compared with those of standard materials such as RuO . Then structural parameters were obtained from a curve fitting analysis. In addition, a fitting analysis used a linear combination of the two standard EXAFS functions of a given elements metal and oxide was applied to determine ratio of metal/oxide in the simulated glass. The redox state of Ru was successfully evaluated from the linear combination fitting results of EXAFS functions. The ratio of metal increased at more reducing atmosphere and at higher temperatures. Chemical form of rhodium oxide in the simulated glass samples was RhO
. Then structural parameters were obtained from a curve fitting analysis. In addition, a fitting analysis used a linear combination of the two standard EXAFS functions of a given elements metal and oxide was applied to determine ratio of metal/oxide in the simulated glass. The redox state of Ru was successfully evaluated from the linear combination fitting results of EXAFS functions. The ratio of metal increased at more reducing atmosphere and at higher temperatures. Chemical form of rhodium oxide in the simulated glass samples was RhO unlike expected Rh
unlike expected Rh O
O . It can be estimated rhodium behaves according with ruthenium when the chemical form is oxide.
. It can be estimated rhodium behaves according with ruthenium when the chemical form is oxide.
Yamagishi, Isao; Odakura, Makoto; Ichige, Yoshiaki; Kuroha, Mitsuhiko; Takano, Masahide; Akabori, Mitsuo; Yoshioka, Masahiro*
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.1113 - 1119, 2015/09
The characteristics of insoluble residues in fine suspension at the Rokkasho Reprocessing Plant were analyzed. The insoluble residues were washed with oxalic acid solution to dissolve zirconium molybdate residues. XRD profiles of unwashed residues showed the presence of a noble metal alloy, zirconium molybdate, and zirconia, but zirconium molybdate was not found after washing. More than 50% of the Sb-125 and Pu in thee residues was washed out as well. The noble metal alloy composed of Mo, Tc, Ru, Rh, and Pd occupied more than 90% of the total weight of 12 elements (Ca, Cr, Fe, Ni, Zr, Mo, Tc, Ru, Rh, Pd, Te, and U) found in the residues. In consideration of the chemical forms of 12 elements, the alloy-to-residue weight ratio was evaluated to be 64% and 78% with and without 18% of an unknown component, respectively.
Hayashi, Hirokazu; Akabori, Mitsuo; Minato, Kazuo
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1331 - 1334, 2015/02
Times Cited Count:0 Percentile:0.01(Chemistry, Analytical)Electrochemical behavior of Am in NaCl-2CsCl melt at 823 K was investigated by transient electrochemical techniques such as cyclic voltammetry and differential pulse voltammetry. The results show that Am(III) ion is reduced to Am metal by a two-step mechanism via Am(II) ion. Formal standard potential of Am(III)/Am(II) and that of Am(II)/Am(0) redox couples have been determined to be -2.73 and -2.97 V vs Cl /Cl
/Cl , respectively.
, respectively.
Okamoto, Yoshihiro; Osugi, Takeshi; Shiwaku, Hideaki; Akabori, Mitsuo
Nihon Genshiryoku Gakkai Wabun Rombunshi, 13(3), p.113 - 118, 2014/09
Transfer behavior of cesium adsorbed on some clay minerals in aqueous solution was investigated by X-ray absorption fine structure (XAFS) analysis of Cs K-edge. The sample was prepared by mixing Cs-adsorbed mineral with another different kind of pure clay mineral in water. The XAFS results of the dried mixture powder were compared with those obtained before the mixing. It was recognized from the XAFS analysis for three kinds of clay minerals; Illite, Kaolinite and Vermiculite, that cesium was transferred from Kaolinite to Illite and vermiculite, and from Illite to Vermiculite. It can be concluded that cesium is transferred to and accumulated in Vermiculite.
Shibata, Hiroki; Hayashi, Hirokazu; Akabori, Mitsuo; Arai, Yasuo; Kurata, Masaki
Journal of Physics and Chemistry of Solids, 75(8), p.972 - 976, 2014/08
Times Cited Count:18 Percentile:59.26(Chemistry, Multidisciplinary)Gibbs free energies of formation of six Ce-Cd intermetallic compounds, CeCd, CeCd , CeCd
, CeCd , CeCd
, CeCd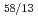 , CeCd
, CeCd and CeCd
and CeCd , were evaluated systematically using electrochemical techniques in the temperature range from 673 to 923 K in the LiCl-KCl-CeCl
, were evaluated systematically using electrochemical techniques in the temperature range from 673 to 923 K in the LiCl-KCl-CeCl -CdCl
-CdCl molten salt bath. The linear dependence of the Gibbs free energies of formation on temperature yields to the enthalpies and entropies of formation of these intermetallic compounds. By extrapolating the molar Gibbs free energy of Ce-Cd intermetallic compounds to the Cd distillation temperature, it was clear that the molar Gibbs free energy of Ce in Ce-Cd intermetallic compounds decreases gradually from CeCd
molten salt bath. The linear dependence of the Gibbs free energies of formation on temperature yields to the enthalpies and entropies of formation of these intermetallic compounds. By extrapolating the molar Gibbs free energy of Ce-Cd intermetallic compounds to the Cd distillation temperature, it was clear that the molar Gibbs free energy of Ce in Ce-Cd intermetallic compounds decreases gradually from CeCd to CeCd
to CeCd and attains to the minimum value at CeCd
and attains to the minimum value at CeCd . This suggests on the Cd distillation from the U-Pu-Ce-Cd alloy that the dissolution of U or Pu into CeCd
. This suggests on the Cd distillation from the U-Pu-Ce-Cd alloy that the dissolution of U or Pu into CeCd should be mostly taken into consideration.
should be mostly taken into consideration.
Suzuki, Chikashi; Nishi, Tsuyoshi; Nakada, Masami; Tsuru, Tomohito; Akabori, Mitsuo; Hirata, Masaru; Kaji, Yoshiyuki
Journal of Physics and Chemistry of Solids, 74(12), p.1769 - 1774, 2013/12
Times Cited Count:14 Percentile:53.53(Chemistry, Multidisciplinary)We investigated the electronic state of a CaF -type (Am,U) mixed oxide using the all-electron full potential linear augmented plane wave method and compared it with those of Am
-type (Am,U) mixed oxide using the all-electron full potential linear augmented plane wave method and compared it with those of Am O
O , AmO
, AmO , UO
, UO , and La
, and La U
U O
O . The valence of Am in the mixed oxide was close to that of Am
. The valence of Am in the mixed oxide was close to that of Am O
O and the valence of U in the mixed oxide was pentavalent. The electronic structure of AmO
and the valence of U in the mixed oxide was pentavalent. The electronic structure of AmO was different from that of Am
was different from that of Am O
O , particularly just above the Fermi level. In addition, the electronic states of Am and U in the mixed oxide were similar to those of trivalent Am and pentavalent U oxides. These electronic states reflected the high oxygen potential of AmO
, particularly just above the Fermi level. In addition, the electronic states of Am and U in the mixed oxide were similar to those of trivalent Am and pentavalent U oxides. These electronic states reflected the high oxygen potential of AmO and the heightened oxygen potential resulting from the addition of Am to UO
and the heightened oxygen potential resulting from the addition of Am to UO and also suggested the occurrence of charge transfer from Am to U in the solid solution process.
and also suggested the occurrence of charge transfer from Am to U in the solid solution process.
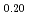 Pu
Pu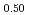 Am
Am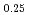 Cm
Cm )O
)O solid solutions
solid solutionsNishi, Tsuyoshi; Takano, Masahide; Akabori, Mitsuo; Arai, Yasuo
Journal of Nuclear Materials, 440(1-3), p.534 - 538, 2013/09
Times Cited Count:2 Percentile:18.63(Materials Science, Multidisciplinary)To clarify the dependence of thermal conductivity on storage time of curium containing oxide, the authors prepared the sintered sample of (Np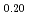 Pu
Pu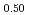 Am
Am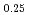 Cm
Cm )O
)O (x = 0.02, 0.04) solid solutions and evaluated the thermal conductivity. The thermal conductivities of (Np
(x = 0.02, 0.04) solid solutions and evaluated the thermal conductivity. The thermal conductivities of (Np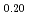 Pu
Pu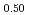 Am
Am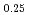 Cm
Cm )O
)O exponentially decreased with increasing storage duration. This result suggested that the degradation of the thermal conductivities was attributed to the accumulation of lattice defects by self-irradiation.
exponentially decreased with increasing storage duration. This result suggested that the degradation of the thermal conductivities was attributed to the accumulation of lattice defects by self-irradiation.
Serizawa, Hiroyuki; Matsunaga, Junji*; Haga, Yoshinori; Nakajima, Kunihisa; Akabori, Mitsuo; Tsuru, Tomohito; Kaji, Yoshiyuki; Kashibe, Shinji*; Oishi, Yuji*; Yamanaka, Shinsuke*
Crystal Growth & Design, 13(7), p.2815 - 2823, 2013/07
Times Cited Count:5 Percentile:42.72(Chemistry, Multidisciplinary)Since the shape of the negative crystal closely relates to the morphology of the crystal habits, the formation and the growth mechanism is important subject in a field of the physical science. Whereas, the negative crystal formed in a large single crystal mass has been arousing interest as an expensive jewelry because of its mysterious appearance and rarity. However, it is difficult to control the shape of this polyhedral cavity embedded in a solid medium arbitrary. Here we report the recent discovery on the growth process of the negative crystal. We found that precipitated helium forms the negative crystal in UO ; the shape changes drastically with the condition of the helium precipitation. The transformation mechanism was discussed in this article. Our investigation implies that the shape of the negative crystal can be arbitrary controlled by controlling the precipitation condition.
; the shape changes drastically with the condition of the helium precipitation. The transformation mechanism was discussed in this article. Our investigation implies that the shape of the negative crystal can be arbitrary controlled by controlling the precipitation condition.
Okamoto, Yoshihiro; Nakada, Masami; Akabori, Mitsuo; Komamine, Satoshi*; Fukui, Toshiki*; Ochi, Eiji*; Nitani, Hiroaki*; Nomura, Masaharu*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 81(7), p.543 - 546, 2013/07
Times Cited Count:7 Percentile:17.93(Electrochemistry)The molten state of simulated high-level waste glass and the behavior of ruthenium element in the melt were investigated by using synchrotron radiation based X-ray imaging technique. Melting, generating and moving of bubbles, condensation and sedimentation of ruthenium element were observed dynamically in continuous 12-bit gray-scale images from the CCD camera. X-ray intensity was obtained easily by digitizing gray-scale values in the image. The existence of ruthenium element is emphasized as a black color in the CCD image at X-ray energy higher than the Ru K-absorption edge. Position sensitive imaging X-ray absorption fine structure (XAFS) measurement was also performed to clarify the chemical state of ruthenium element in the melt.
Hayashi, Hirokazu; Hagiya, Hiromichi; Kim, S.-Y.*; Morita, Yasuji; Akabori, Mitsuo; Minato, Kazuo
Journal of Radioanalytical and Nuclear Chemistry, 296(3), p.1275 - 1286, 2013/06
Times Cited Count:4 Percentile:32.48(Chemistry, Analytical) Cm was separated and recovered as an oxalate from
Cm was separated and recovered as an oxalate from  Cm-
Cm- Pu mixed oxide which had been
Pu mixed oxide which had been  Cm oxide sample prepared 40 years ago. Plutonium ions were removed from the solution prepared by dissolution of
Cm oxide sample prepared 40 years ago. Plutonium ions were removed from the solution prepared by dissolution of  Cm-
Cm- Pu mixed oxide in nitric acid, by using an anion exchange resin column. Curium oxalate, a precursor compound of curium oxide, was prepared from the purified curium solution and supplied for the syntheses and measurements of the thermochemical properties of curium compounds.
Pu mixed oxide in nitric acid, by using an anion exchange resin column. Curium oxalate, a precursor compound of curium oxide, was prepared from the purified curium solution and supplied for the syntheses and measurements of the thermochemical properties of curium compounds.
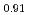 Cm
Cm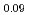 )O
)O
Nishi, Tsuyoshi; Takano, Masahide; Akabori, Mitsuo; Arai, Yasuo
Journal of Nuclear Materials, 433(1-3), p.531 - 533, 2013/02
Times Cited Count:6 Percentile:44.02(Materials Science, Multidisciplinary)To clarify the storage duration dependence of the thermal conductivity of MA containing oxide fuel, the thermal diffusivity of (Pu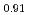 Cm
Cm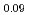 )O
)O was measured at 473, 523 and 573 K by a laser flash method using the sample stored for 48, 264, 504, and 960 h. The heat capacity was measured by a drop calorimetry to derive the thermal conductivity. It was confirmed that the degradation of the thermal conductivity was attributed to the accumulation of lattice defects caused by self-irradiation, because the storage duration dependence of the thermal conductivity could be approximated by the equation used for self-irradiation lattice expansion model.
was measured at 473, 523 and 573 K by a laser flash method using the sample stored for 48, 264, 504, and 960 h. The heat capacity was measured by a drop calorimetry to derive the thermal conductivity. It was confirmed that the degradation of the thermal conductivity was attributed to the accumulation of lattice defects caused by self-irradiation, because the storage duration dependence of the thermal conductivity could be approximated by the equation used for self-irradiation lattice expansion model.
Okamoto, Yoshihiro; Nakada, Masami; Akabori, Mitsuo; Komamine, Satoshi*; Fukui, Toshiki*; Ochi, Eiji*; Nitani, Hiroaki*; Nomura, Masaharu*
Proceedings of 4th Asian Conference on Molten Salt Chemistry and Technology & 44th Symposium on Molten Salt Chemistry, Japan, p.47 - 52, 2012/09
The molten state of the simulated high-level waste glass and the behavior of ruthenium element in the melt were investigated by using synchrotron radiation based X-ray imaging technique. Melting, generating and moving of bubbles, condensation and sedimentation of ruthenium element were observed dynamically in continuous 12-bit gray-scale images from the CCD camera. The existence of ruthenium in the X-ray CCD image was emphasized over the energy of Ru K-absorption edge. X-ray intensity was obtained easily by digitalizing gray-scale values in the image. Position sensitive imaging X-ray absorption fine structure (XAFS) measurement was performed to clarify the chemical state of ruthenium element in the melt.
Okamoto, Yoshihiro; Nakada, Masami; Akabori, Mitsuo; Shiwaku, Hideaki; Komamine, Satoshi*; Fukui, Toshiki*; Ochi, Eiji*; Nitani, Hiroaki*; Nomura, Masaharu*
Nihon Genshiryoku Gakkai Wabun Rombunshi, 11(2), p.127 - 132, 2012/06
Distribution and the chemical state of Ru element in the simulated high-level waste glass were examined by using the synchrotron radiation based X-ray imaging technique. In this technique, a direct X-ray CCD camera is used in place of an ion chamber. Position sensitive X-ray absorption spectra were obtained by analyzing gray scale in images of the X-ray CCD camera. At first, we measured a test sample containing RuO and Ru metal powder. We successfully obtained information on the Ru distribution in the sample. In addition, the chemical state (oxide or metal ?) of each small Ru-rich spot was evaluated by the corresponding position sensitive XAFS spectrum. The imaging XAFS technique was applied to some simulated high-level waste glass samples. The Ru distribution of the glass sample and their chemical state were confirmed by image analyses. It can be seen that Ru element scattered in the glass sample exists as oxide RuO
and Ru metal powder. We successfully obtained information on the Ru distribution in the sample. In addition, the chemical state (oxide or metal ?) of each small Ru-rich spot was evaluated by the corresponding position sensitive XAFS spectrum. The imaging XAFS technique was applied to some simulated high-level waste glass samples. The Ru distribution of the glass sample and their chemical state were confirmed by image analyses. It can be seen that Ru element scattered in the glass sample exists as oxide RuO .
.
Suzuki, Chikashi; Nishi, Tsuyoshi; Nakada, Masami; Akabori, Mitsuo; Hirata, Masaru; Kaji, Yoshiyuki
Journal of Physics and Chemistry of Solids, 73(2), p.209 - 216, 2012/02
Times Cited Count:26 Percentile:69.41(Chemistry, Multidisciplinary)The authors investigated theoretically core-hole effects on X-ray absorption near-edge structures (XANES) of Np and Am L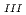 in neptunium dioxide (NpO
in neptunium dioxide (NpO ) and americium dioxide (AmO
) and americium dioxide (AmO ) with CaF
) with CaF -type crystal lattices using the all-electron full-potential linearized augmented plane-wave (FP-LAPW) method. The peak creation mechanism of XANES was shown by examining the electronic structures of these oxides, which indicated that core-hole screening was more marked for AmO
-type crystal lattices using the all-electron full-potential linearized augmented plane-wave (FP-LAPW) method. The peak creation mechanism of XANES was shown by examining the electronic structures of these oxides, which indicated that core-hole screening was more marked for AmO than for NpO
than for NpO because of the difference in the charge transfer between these oxides. Furthermore, the results of charge density analysis suggested that the white line was assigned to the quasi-bound state composed of the localized Np d or Am d components and O components, and that the tail structure was created as a result of delocalized standing waves between the Np or Am atoms.
because of the difference in the charge transfer between these oxides. Furthermore, the results of charge density analysis suggested that the white line was assigned to the quasi-bound state composed of the localized Np d or Am d components and O components, and that the tail structure was created as a result of delocalized standing waves between the Np or Am atoms.
Arai, Yasuo; Serizawa, Hiroyuki; Nakajima, Kunihisa; Takano, Masahide; Sato, Isamu; Katsuyama, Kozo; Akie, Hiroshi; Suzuki, Motoe; Shirasu, Noriko; Haga, Yoshinori; et al.
Proceedings of International Conference on Toward and Over the Fukushima Daiichi Accident (GLOBAL 2011) (CD-ROM), 8 Pages, 2011/12
High amount of He is generated in MA-bearing fuel during irradiation and storage periods compared with that in U or U-Pu fuel. Laboratory scale experiments, post irradiation examinations and modeling study were carried out in order to understand the He behavior in MA-bearing oxide fuel. Diffusion characteristics of He in single-crystal UO were investigated by the Knudsen effusion mass spectrometry. Effects of the He accumulation on lattice and bulk expansion of oxide pellets were examined by use of alpha-decay of
were investigated by the Knudsen effusion mass spectrometry. Effects of the He accumulation on lattice and bulk expansion of oxide pellets were examined by use of alpha-decay of  Cm. Post irradiation examinations of 0.5%Am-MOX fuel irradiated at a fast test reactor JOYO were carried out, concentrating on the He behavior in the fuel pellets. A model describing the He behavior in MA-MOX fuel was constructed based on the principle processes, such as generation, diffusion, equilibrium and release to outer gaseous phase. By use of the model as a subroutine of a conventional fuel behavior analysis code, the He behavior in MA-MOX fuel for fast reactors was simulated.
Cm. Post irradiation examinations of 0.5%Am-MOX fuel irradiated at a fast test reactor JOYO were carried out, concentrating on the He behavior in the fuel pellets. A model describing the He behavior in MA-MOX fuel was constructed based on the principle processes, such as generation, diffusion, equilibrium and release to outer gaseous phase. By use of the model as a subroutine of a conventional fuel behavior analysis code, the He behavior in MA-MOX fuel for fast reactors was simulated.
 Pu
Pu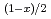 Am
Am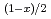 )N solid solutions
)N solid solutionsNishi, Tsuyoshi; Takano, Masahide; Akabori, Mitsuo; Arai, Yasuo
Proceedings of International Conference on Toward and Over the Fukushima Daiichi Accident (GLOBAL 2011) (CD-ROM), 6 Pages, 2011/12
The thermal conductivity of Zr-based minor actinide (MA) nitride solid solutions is important for designing subcritical cores in nitride-fueled ADS. However, there have been no experimental data on the thermal conductivities of Zr-based nitride solid solutions containing MA. In this study, the authors prepared sintered samples of (Zr Pu
Pu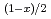 Am
Am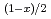 )N (x=0.0, 0.58, 0.80) solid solutions. The thermal diffusivity and heat capacity of (Zr
)N (x=0.0, 0.58, 0.80) solid solutions. The thermal diffusivity and heat capacity of (Zr Pu
Pu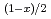 Am
Am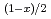 )N solid solutions were measured using a laser flash method and drop calorimetry, respectively. Thermal conductivities were determined from the measured thermal diffusivities, heat capacities and bulk densities over a temperature range of 473 to 1473 K. Moreover, in order to help to promote the design study of nitride-fueled ADS, the thermal conductivity of the (Zr
)N solid solutions were measured using a laser flash method and drop calorimetry, respectively. Thermal conductivities were determined from the measured thermal diffusivities, heat capacities and bulk densities over a temperature range of 473 to 1473 K. Moreover, in order to help to promote the design study of nitride-fueled ADS, the thermal conductivity of the (Zr Pu
Pu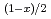 Am
Am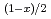 )N solid solutions were fitted to an equation using the least squares method.
)N solid solutions were fitted to an equation using the least squares method.
 Am
Am )O
)O
Nishi, Tsuyoshi; Nakada, Masami; Suzuki, Chikashi; Shibata, Hiroki; Okamoto, Yoshihiro; Akabori, Mitsuo; Hirata, Masaru
Journal of Nuclear Materials, 418(1-3), p.311 - 312, 2011/11
Times Cited Count:20 Percentile:81.61(Materials Science, Multidisciplinary)The XAFS measurements at U-L and Am-L
and Am-L absorption edge of (U
absorption edge of (U Am
Am )O
)O were performed in transmission mode. Moreover, to clarify the valence state of Am in (U,Am)O
were performed in transmission mode. Moreover, to clarify the valence state of Am in (U,Am)O , the XANES spectrum of Am-L
, the XANES spectrum of Am-L absorption edge of (U
absorption edge of (U Am
Am )O
)O was verified using those of Am-L
was verified using those of Am-L absorption edge of AmO
absorption edge of AmO and Am
and Am O
O . It was found that the XANES spectrum of the Am-L
. It was found that the XANES spectrum of the Am-L edge of (U
edge of (U Am
Am )O
)O is in good accordance with that of Am
is in good accordance with that of Am O
O . Thus, Am in (U
. Thus, Am in (U Am
Am )O
)O is almost trivalent state.
is almost trivalent state.
 lattice and bulk expansion from self-irradiation damage
lattice and bulk expansion from self-irradiation damageTakano, Masahide; Akabori, Mitsuo; Arai, Yasuo
Journal of Nuclear Materials, 414(2), p.174 - 178, 2011/07
Times Cited Count:9 Percentile:57.01(Materials Science, Multidisciplinary)In order to investigate the effect of self-irradiation damage and accumulation of He on the oxide fuel pellet containing minor actinides, the expansion and annealing behavior of (Pu Cm
Cm )O
)O lattice and bulk were examined comparatively. Since the lattice and bulk expansion at room temperature showed the similar dependence on the storage duration, the main factor of bulk swelling was found to be the lattice expansion due to the generation of point defects. The lattice parameter recovered to the undamaged value by annealing at 1429 K for 2 h, whereas the bulk expanded again by annealing at 1433 K and did not recover to the undamaged value at all. On the micrographs of fracture surface of the annealed pellet, formation of gas bubbles along grain boundaries was confirmed. The accumulated He atoms migrated to the grain boundaries and formed gas bubbles, which caused the He gas swelling of the pellet.
lattice and bulk were examined comparatively. Since the lattice and bulk expansion at room temperature showed the similar dependence on the storage duration, the main factor of bulk swelling was found to be the lattice expansion due to the generation of point defects. The lattice parameter recovered to the undamaged value by annealing at 1429 K for 2 h, whereas the bulk expanded again by annealing at 1433 K and did not recover to the undamaged value at all. On the micrographs of fracture surface of the annealed pellet, formation of gas bubbles along grain boundaries was confirmed. The accumulated He atoms migrated to the grain boundaries and formed gas bubbles, which caused the He gas swelling of the pellet.