Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Takeda, Tetsuaki*; Inagaki, Yoshiyuki; Aihara, Jun; Aoki, Takeshi; Fujiwara, Yusuke; Fukaya, Yuji; Goto, Minoru; Ho, H. Q.; Iigaki, Kazuhiko; Imai, Yoshiyuki; et al.
High Temperature Gas-Cooled Reactors; JSME Series in Thermal and Nuclear Power Generation, Vol.5, 464 Pages, 2021/02
As a general overview of the research and development of a High Temperature Gas-cooled Reactor (HTGR) in JAEA, this book describes the achievements by the High Temperature Engineering Test Reactor (HTTR) on the designs, key component technologies such as fuel, reactor internals, high temperature components, etc., and operational experience such as rise-to-power tests, high temperature operation at 950 C, safety demonstration tests, etc. In addition, based on the knowledge of the HTTR, the development of designs and component technologies such as high performance fuel, helium gas turbine and hydrogen production by IS process for commercial HTGRs are described. These results are very useful for the future development of HTGRs. This book is published as one of a series of technical books on fossil fuel and nuclear energy systems by the Power Energy Systems Division of the Japan Society of Mechanical Engineers.
C, safety demonstration tests, etc. In addition, based on the knowledge of the HTTR, the development of designs and component technologies such as high performance fuel, helium gas turbine and hydrogen production by IS process for commercial HTGRs are described. These results are very useful for the future development of HTGRs. This book is published as one of a series of technical books on fossil fuel and nuclear energy systems by the Power Energy Systems Division of the Japan Society of Mechanical Engineers.
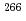 Bh in the
Bh in the  Cm(
Cm(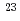 Na,5
Na,5 )
)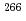 Bh reaction and its decay properties
Bh reaction and its decay propertiesHaba, Hiromitsu*; Fan, F.*; Kaji, Daiya*; Kasamatsu, Yoshitaka*; Kikunaga, Hidetoshi*; Komori, Yukiko*; Kondo, Narumi*; Kudo, Hisaaki*; Morimoto, Koji*; Morita, Kosuke*; et al.
Physical Review C, 102(2), p.024625_1 - 024625_12, 2020/08
Times Cited Count:6 Percentile:59.56(Physics, Nuclear) KM1
KM1Okazaki, Nobuo; Tamada, Taro; Feese, M. D.*; Kato, Masaru*; Miura, Yutaka*; Komeda, Toshihiro*; Kobayashi, Kazuo*; Kondo, Keiji*; Blaber, M.*; Kuroki, Ryota
Protein Science, 21(4), p.539 - 552, 2012/04
Times Cited Count:3 Percentile:6.67(Biochemistry & Molecular Biology); Sakakibara, Yasuhide; Nishida, Masaaki; kondou; Nagata, Takashi
ProVISION, Fall(39), p.30 - 37, 2003/00
None
Yamanouchi, Sadamu; Kuwajima, Y.; Namekawa, Takashi; Inui, T.; Kondo, M.; Tani, Y.; Usui, Keiji; Nagai, Shuichiro
PNC TN9410 85-138, 109 Pages, 1985/03
The JOYO MK-I core fuel subassembly PPJX13 was irradiated in JOYO MK-I core (location; 000) from 50 MW test through 75 MW 6th cycle. The average burnup was about 40,100 MWD/MTM. Five fuel pins was selected in the subassembly. Destructive examination items were metallographic examination (fuel and cladding), micro-hardness measurement, cladding density measurement and burnup measurement. The following results were obtained; (1) Central void of about 250Mm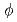 , columner grain region, equiaxed region (gas bubble region) and densified equiaxed region was formed by fuel restrusting. (2) Fuel and Cladding residual gap width were 20
, columner grain region, equiaxed region (gas bubble region) and densified equiaxed region was formed by fuel restrusting. (2) Fuel and Cladding residual gap width were 20  m
m  89
89  m. Fuel-cladding chemical interaction was 13
m. Fuel-cladding chemical interaction was 13  m at the maximum cladding temperature. (3) Carbide precipitation along the grain boundary and twinning zone were observed at high temperature position in cladding. Cladding micro-hardness indicated high value at low temperature position. (4) The cladding density change of 1.10% were observed in R material. (5) Burnup measurement by
m at the maximum cladding temperature. (3) Carbide precipitation along the grain boundary and twinning zone were observed at high temperature position in cladding. Cladding micro-hardness indicated high value at low temperature position. (4) The cladding density change of 1.10% were observed in R material. (5) Burnup measurement by 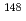 Nd isotope analysis using mass spectrometer showed 5.22 atom % at the center position of the center fuel pin.
Nd isotope analysis using mass spectrometer showed 5.22 atom % at the center position of the center fuel pin.
Takai, Yu*; Kondo, Manabu*; Iwamoto, Yukiharu*; Yasuda, Kazunori*; Sogo, Motosuke*; Tanaka, Masaaki; Yamano, Hidemasa
no journal, ,
In the present study, velocity distributions were measured using LDV under 320000 of Reynolds number in an experimental facility, where a swirling flow entered a 90 degree bend.
Kondo, Manabu*; Takai, Yu*; Iwamoto, Yukiharu*; Yasuda, Kazunori*; Sogo, Motosuke*; Yamano, Hidemasa; Tanaka, Masaaki
no journal, ,
In the present study, velocity distributions were measured using LDV under 320000 of Reynolds number in an experimental facility, where a swirling flow entered to a 90-degree bend.
Okazaki, Nobuo; Tamada, Taro; Miura, Yutaka*; Feese, M. D.*; Kato, Masaru*; Komeda, Toshihiro*; Takehara, Kyoko*; Kobayashi, Kazuo*; Kondo, Keiji*; Kuroki, Ryota
no journal, ,
The crystal structure of glycosyltrehalose synthase (GTSase) from the hyperthermophilic archaeum Sulfolobus shibatae DSM5389 has been determined to 2.3 A resolution by X-ray crystallography. GTSase converts the glucosidic bond between the last two glucose residues of amylose from an alpha-1,4 bond to an alpha-1,1 bond, making a non-reducing glycosyl trehaloside in the first step of the biosynthesis of trehalose. The structure of GTSase can be divided into five domains. The central domain has the (beta/alpha)8 barrel fold which is conserved in the alpha-amylase family as the catalytic domain. Three invariant catalytic carboxylic amino acids in the alpha-amylase family are also found in GTSase at positions Asp241, Glu269 and Asp460 in the (beta/alpha)8 domain. Our previous study with KM1-GTSase has been shown that the maltooligosaccharides are converted to glycosyltrehalose by an intramolecular transglycosylation mechanism.
Iwai, Yasunori; Kondo, Akiko*; Edao, Yuki; Sato, Katsumi; Kubo, Hitoshi*; Oshima, Yusuke*
no journal, ,
Effect of halogenated gas on detritiation efficiency of the detritiation system has been investigated taking an event of off normal event such as fire into consideration. Concerning the activity of platinum catalyst for oxidation of tritium, we have evaluated the steep decrease in activity of platinum catalyst in the presence of halogenated gas. In order to avoid the steep decrease in activity, a noble catalyst alloyed with platinum and palladium showed an outstanding proof. In addition, the halogenated acid produced over catalyst surface affects the activity of catalyst. As for water absorber, a molecular sieve decreased its water absorbing capacity in the presence of halogenated gas.