Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nomizu, Daiki; Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Katsuta, Shoichi*
Journal of Radioanalytical and Nuclear Chemistry, 331(3), p.1483 - 1493, 2022/03
Times Cited Count:4 Percentile:76.47(Chemistry, Analytical)We studied the successive formation of water soluble DGA (diglycolamide) and DOODA (dioxaoctanediamide) for the mutual separation of Ln in this extraction system. TODGA (tetraoctyl-diglycolamide) and DOODA(C8) (tetraoctyl-dioxaoctanediamide) have the opposite trend to extract light and heavy Ln through Ln-patterns. Metal-complexes of two folding Ln ions with water-soluble DOODA and three folding with DGA are found and their observed formation constants are calculated. The suitable separation condition (aqueous phase: 30 mM DOODA(C2) in 1 M nitric acid, organic phase: 0.1 M TODGA in n-dodecane) of multi-stage extraction (10  10) is conducted. From the present work, it is clear that La, Pr and Nd are mainly present in aqueous phase, instead Sm-Dy exist in the organic phase.
10) is conducted. From the present work, it is clear that La, Pr and Nd are mainly present in aqueous phase, instead Sm-Dy exist in the organic phase.
Matsumiya, Masahiko*; Nomizu, Daiki*; Tsuchida, Yusuke*; Sasaki, Yuji
Hydrometallurgy, 199, p.105539_1 - 105539_8, 2021/02
Times Cited Count:4 Percentile:32.7(Metallurgy & Metallurgical Engineering)The synergistic solvent extraction of lanthanide(III) with mixtures of di-(2-ethylhexyl)phosphoric acid (D2EHPA, A) and monoisodecyl phosphoric acid (MIDPA, B) in phosphonium-based ionic liquid was investigated. In the case of D2EHPA or MIDPA single extractant system, Ln(III) (Ln = Pr and Nd) was extracted as [LnA HA] or [LnB
HA] or [LnB HB], respectively, the extracted species of Tb(III) or Dy(III) were determined by slope analysis. According to the equilibrium constants (
HB], respectively, the extracted species of Tb(III) or Dy(III) were determined by slope analysis. According to the equilibrium constants (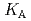 ,
, 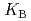 and
and 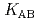 ) and the formation constants (
) and the formation constants (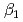 ,
, 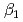 and
and 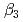 ), it was found that the extracted complex [TbHA
), it was found that the extracted complex [TbHA B
B or [DyHA
or [DyHA B
B ] was more stable than [LnA
] was more stable than [LnA HA] or [LnB
HA] or [LnB HB]. The synergistic extraction effects were investigated to study the possibility of separating Dy(III) from Pr(III) and Nd(III) according to their separation factors.
HB]. The synergistic extraction effects were investigated to study the possibility of separating Dy(III) from Pr(III) and Nd(III) according to their separation factors.
 -diketone extractant and a neutral ligand
-diketone extractant and a neutral ligandMatsumiya, Masahiko*; Nomizu, Daiki*; Tsuchida, Yusuke*; Sasaki, Yuji
Solvent Extraction and Ion Exchange, 39(7), p.764 - 784, 2021/00
Times Cited Count:4 Percentile:22.03(Chemistry, Multidisciplinary)We investigated the solvent extraction of four rare earth (RE) elements (Pr, Nd, Tb, and Dy) from Nd-Fe-B magnets using mixtures of 1-(2-thienyl)-4,4,4,-trifluoro-1,3-butanedione (Htta) or 4,4,4-trifluoro-1-phenyl-1,3-butanedione (Hbfa) chelating extractants and tri-n-octylphosphine oxide (TOPO) neutral ligand in phosphonium based ionic liquids. A synergistic effect was observed for the extraction of the RE elements with the combination of extractant and neutral ligand. The separation of Tb(III) and Dy(III) from other RE(III) components was performed with seven extraction cycles.
Nomizu, Daiki; Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Katsuta, Shoichi*
no journal, ,
In our group, the mutual separation of lanthanides by solvent extraction method is conducted. We focus on two phases in solvent extraction, the hydrophilic and lipophilic reagents are employed in both phases. Namely, using the condition of TODGA in organic phase and DOODA(C2, dioxaoctane-diamide) in aqueous phase or DOODA(C8) in organic phase and TEDGA in aqueous phase, the distribution ratios of Ln are compared. Using these results, the batch-wise multi stage extractions are performed in order to separate light and heavy Ln.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Nomizu, Daiki*; Tsuchida, Yusuke*; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*; Shimosaka, Takahiro*; Suzuki, Tatsuya*
no journal, ,
In order to enlarge the separation factors between trivalent actinide (An) and lanthanide (Ln), which show similar chemical behavior, the use of water-soluble ligand has been studied. In this work, ten of amides and carboxylic acids are employed in TODGA extraction system and their properties are compared. It is noted that complexing ability of amide with Ln is higher than that with carboxylic acid under pH 2, if both reagents have the same central frame. Classifying 14 lanthanides into four groups (La-Nd, Sm-Gd, Gd-Ho, and Er-Lu), the difference of their complexing reactions in each group and the reaction of An will be discussed.