Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
*; Sato, Haruo; *
JNC TN8400 99-059, 59 Pages, 1999/10
Organic acids in groundwater are considered to form complexes and increase the solubility of radionuclides released from vitrified waste in a high-level radioactive waste (HLW) repository. To ivestigate whether the solubility of samarium (Sm) is influenced by organic substances, we measured Sm solubility in the presence of different organic substances and compared those values with results from thermodynamic predictions. Humic acid (Aldrich) is commercially available and soluble organic matter originated from bentonite were used as organic substances in this study. Consequently, the solubility of Sm showed a tendency to apparently increase with icreasing the concentration of humic acid, but in the presence of carbonate, thermodynamic predictions suggested that the dominant species are carbonate complexes and that the effect of organic substances are less than that of carbonate. Based on total organic carbon (TOC), the increase of Sm solubility measured with humic acid (Aldrich) was more significant than that in the case with soluble organic matter originated from bentonite. Since bentonite is presumed to include also simple organic matters of which stability constant for forming complexes is low, the effect of soluble organic matter originated from bentonite on the solubility of Sm is eonsidered to be less effective than that of humic acid (Aldrieh). Experimental values were compared with model prediction, propsed by Kim, based on data measured in a low pH region. Tentatively we calculated the increase in Sm solubility assuming complexation with humic acid. Trial calculations were carried out on the premise that the complexation reaction of metal ion with humic acid is based on neutralization process by 1-1 complexation. In this process, it was assumed that one metal ion coordinates with one unit of complexation sites which number of proton exchange sites is equal to ionic charge. Consequently, Kim's model indicated that carbonate complexes should be dominant ...
; Yui, Mikazu; *
JNC TN8400 99-080, 19 Pages, 1999/07
Filtration behavior of organic substance through a compacted bentonite was investigated. Na-type bentonite containing 30wt% of quartz sand was compacted in a column and the dry density was adjusted to be 1.6 g/cm . Polyacrylic acid solution (including three types of polyacrynclic acid, average molecular weight 2,100, 15,000 and 450,000) was prepared and was passed through the compacted bentonite. Molecular weight distributions of polyacrylic acid in the effluent solution were analysed by GPC (Gel Permeation Chromatography). A batch type experiment was also carried out in order to examine a sorption behavior of these organic substances onto the surfaces of grains of the bentonite. The results indicated that the smaller size polyacrylic acid (molecular weight
. Polyacrylic acid solution (including three types of polyacrynclic acid, average molecular weight 2,100, 15,000 and 450,000) was prepared and was passed through the compacted bentonite. Molecular weight distributions of polyacrylic acid in the effluent solution were analysed by GPC (Gel Permeation Chromatography). A batch type experiment was also carried out in order to examine a sorption behavior of these organic substances onto the surfaces of grains of the bentonite. The results indicated that the smaller size polyacrylic acid (molecular weight  100,000) was passed through the compacted bentonite. On the other hand, the larger size polyacrylic acid (molecular weight
100,000) was passed through the compacted bentonite. On the other hand, the larger size polyacrylic acid (molecular weight 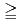 100,000) was mostly filtrated by the compacted bentonite. The batch type sorption tests clarified that the polyacrylic acid did not sorb onto the surfaces of minerals constituting the bentonite. Therefore it was suggested that the larger size molecules (
100,000) was mostly filtrated by the compacted bentonite. The batch type sorption tests clarified that the polyacrylic acid did not sorb onto the surfaces of minerals constituting the bentonite. Therefore it was suggested that the larger size molecules (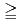 100,000) of organic substances could be predominantly filtrated by the microstructure of the compacted bentonite.
100,000) of organic substances could be predominantly filtrated by the microstructure of the compacted bentonite.
*; Sato, Haruo
PNC TN8410 98-102, 8 Pages, 1998/10
Bentonite-water reaction experiments were carried out by a batch method to estimate an amount of soluble organic matter originated from bentonite which is permeable in compacted bentonite. Bentonite (Kunigel V1) was equilibrated with distilled water for  180days at a liquid/solid ratio of 0.01m
180days at a liquid/solid ratio of 0.01m /kg at room temperature under aerobic conditions. The suspensions were then filtered by an ultrafilter of molecular weight cut-off 10,000 which pore size is approximately equivalent to the interlayer aperture of compacted bentonite. The concentration of organic carbon in the filtrate was analyzed. It was found that the concentration of organic carbon in the equilibrated solutions was between 3 and 4ppm. Since the bentonite used contains 0.31-0.34 wt% organic carbons, it is estimated that the amount of soluble organic matter, which can permeate through compacted bentonite, corresponds to about 1 wt% of total organic matter in the bentonite.
/kg at room temperature under aerobic conditions. The suspensions were then filtered by an ultrafilter of molecular weight cut-off 10,000 which pore size is approximately equivalent to the interlayer aperture of compacted bentonite. The concentration of organic carbon in the filtrate was analyzed. It was found that the concentration of organic carbon in the equilibrated solutions was between 3 and 4ppm. Since the bentonite used contains 0.31-0.34 wt% organic carbons, it is estimated that the amount of soluble organic matter, which can permeate through compacted bentonite, corresponds to about 1 wt% of total organic matter in the bentonite.