Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
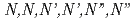 -hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cell
-hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsubata, Yasuhiro
Solvent Extraction Research and Development, Japan, 31(1), p.1 - 11, 2024/00
A demonstration test was performed to separate minor actinides (MA; Am and Cm) by 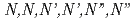 -hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm
-hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm in
in  -dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were
-dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were  99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Suzuki, Hideya*
Journal of Nuclear Science and Technology, 11 Pages, 2023/11
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)The mutual separation of Am and Cm is conducted using an alkyl-diamide amine (ADAAM) extractant. ADAAM exhibits extremely high separation factor with respect to Am and Cm separation (5.9) in a nitric acid- -dodecane system. The batch-wise multistage extractions are performed using a system containing 0.2 M ADAAM and 1.5 M nitric acid. In this multistage extraction, an organic solvent give 96.5% and 1.06% yields of Am and Cm. After the mutual separation of Am and Cm, an additional extraction step is included to reduce the volumes of these aqueous and organic phases. Taking these steps, Am and Cm can be recovered in just two or three stages in the aqueous phases.
-dodecane system. The batch-wise multistage extractions are performed using a system containing 0.2 M ADAAM and 1.5 M nitric acid. In this multistage extraction, an organic solvent give 96.5% and 1.06% yields of Am and Cm. After the mutual separation of Am and Cm, an additional extraction step is included to reduce the volumes of these aqueous and organic phases. Taking these steps, Am and Cm can be recovered in just two or three stages in the aqueous phases.
Suzuki, Hideya*; Ban, Yasutoshi
Analytical Sciences, 39(8), p.1341 - 1348, 2023/08
Times Cited Count:2 Percentile:76.52(Chemistry, Analytical)The Japan Atomic Energy Agency (JAEA) has proposed the Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation (SELECT) process by solvent extraction as a new separation technology to recover minor actinides (MA) from high-level liquid waste (HLLW) produced by spent fuel reprocessing. The MA separation in the SELECT process comprises the batch recovery of MA and rare earths (RE) from HLLW, MA/RE separation, and Am/Cm separation. Three highly practical extractants are used in the MA separation. Furthermore, this flow configuration facilitates the preparation of nitric acid concentrations in the aqueous phase. However, the separation factor between Cm and Nd in the MA/RE separation is small (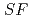
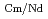 = 2.5), requiring many extraction stages for continuous extraction in a mixer-settler. Therefore, this study investigated the separation of only Am from an aqueous nitric acid solution containing MA (Am and Cm) and RE using an organic phase mixed with two extractants alkyl diamideamine with 2-ethylhexyl alkyl chains (ADAAM(EH)) and hexa-n-octylnitrilotriacetamide (HONTA) used in the SELECT process. Under high-concentration nitric acid conditions, Am and La, Ce, Pr, Nd (light lanthanides) were extracted in the ADAAM(EH) + HONTA mixed solvent, whereas Cm, medium, and heavy lanthanides, and Y were partitioned in the aqueous phase. Subsequently, only light lanthanides could be back extracted from the ADAAM(EH) + HONTA mixture solvent containing Am and light lanthanides in low nitric acid concentrations. Furthermore, Am could be easily stripped with 0.2 M or 5 M nitric acid. This method does not require the mutual separation of Cm and Nd, which have low separation factors. Am can be efficiently separated by one extraction and two back-extractions, reducing the number of steps in the SELECT process.
= 2.5), requiring many extraction stages for continuous extraction in a mixer-settler. Therefore, this study investigated the separation of only Am from an aqueous nitric acid solution containing MA (Am and Cm) and RE using an organic phase mixed with two extractants alkyl diamideamine with 2-ethylhexyl alkyl chains (ADAAM(EH)) and hexa-n-octylnitrilotriacetamide (HONTA) used in the SELECT process. Under high-concentration nitric acid conditions, Am and La, Ce, Pr, Nd (light lanthanides) were extracted in the ADAAM(EH) + HONTA mixed solvent, whereas Cm, medium, and heavy lanthanides, and Y were partitioned in the aqueous phase. Subsequently, only light lanthanides could be back extracted from the ADAAM(EH) + HONTA mixture solvent containing Am and light lanthanides in low nitric acid concentrations. Furthermore, Am could be easily stripped with 0.2 M or 5 M nitric acid. This method does not require the mutual separation of Cm and Nd, which have low separation factors. Am can be efficiently separated by one extraction and two back-extractions, reducing the number of steps in the SELECT process.
Uchino, Seiko*; Narita, Hirokazu*; Kita, Keisuke*; Suzuki, Hideya*; Matsumura, Tatsuro; Naganawa, Hirochika*; Sakaguchi, Koichi*; Oto, Keisuke*
Solvent Extraction Research and Development, Japan, 30(1), p.39 - 46, 2023/00
The extraction of trivalent rare earth ions (RE ) from HNO
) from HNO solution using a triamide amine, tris(N,N-di-2-ethylhexyl-ethylamide)amine (DEHTAA), was conducted, and the extraction mechanism was estimated from extraction behavior of HNO
solution using a triamide amine, tris(N,N-di-2-ethylhexyl-ethylamide)amine (DEHTAA), was conducted, and the extraction mechanism was estimated from extraction behavior of HNO and RE
and RE and the relationship between atomic number and extraction percentages (E%) for RE
and the relationship between atomic number and extraction percentages (E%) for RE . A DEHTAA molecule dominantly formed a DEHTAA HNO
. A DEHTAA molecule dominantly formed a DEHTAA HNO at 1.0 M HNO
at 1.0 M HNO and a DEHTAA(HNO
and a DEHTAA(HNO )
) at 6.0 M HNO
at 6.0 M HNO in the acid-equilibrated organic phase. This would provide the unique dependence of E% for the light RE
in the acid-equilibrated organic phase. This would provide the unique dependence of E% for the light RE on the HNO
on the HNO concentration, in which the E% value had a minimum and maximum at
concentration, in which the E% value had a minimum and maximum at  0.5 M and
0.5 M and  2 M HNO
2 M HNO , respectively. The results of the slope analyses for the distribution ratios for RE
, respectively. The results of the slope analyses for the distribution ratios for RE suggested that the dominant RE
suggested that the dominant RE complex was RE(NO
complex was RE(NO )
) DEHTAA(DEHTAA HNO
DEHTAA(DEHTAA HNO ) at 1.0 M HNO
) at 1.0 M HNO . The E% for RE
. The E% for RE decreased from La
decreased from La to Lu
to Lu at 1.0 M HNO
at 1.0 M HNO ; on the other hand, those increased from La
; on the other hand, those increased from La to Nd
to Nd at 0.25 M and from La
at 0.25 M and from La to Sm
to Sm and 6.0 M HNO
and 6.0 M HNO .
.
Sakamoto, Atsushi; Kibe, Satoshi*; Kawanobe, Kazunori*; Fujisaku, Kazuhiko*; Sano, Yuichi; Takeuchi, Masayuki; Suzuki, Hideya*; Tsubata, Yasuhiro; Ban, Yasutoshi; Matsumura, Tatsuro
JAEA-Research 2021-003, 30 Pages, 2021/06
Japan Atomic Energy Agency has been developing a solvent extraction process called SELECT to recover minor actinides (MA) from spent nuclear fuel. In the SELECT process, TDdDGA, HONTA, and ADAAM are used as the extractants for MA + Ln corecovery, MA/Ln separation and Am/Cm separation, respectively. These extractants do not contain phosphorus (P), and consist of carbon (C), hydrogen (H), oxygen (O), and nitrogen (N). In this study, in order to give beneficial information for designing flowsheet, the mass transfer coefficients of Ln between HNO solution and TDdDGA or HONTA / n-dodecane solvent were evaluated by the single drop technique. Prior to the evaluation of mass transfer coefficient, we had optimized the structure of the single drop apparatus to improve accuracy of the measurement. Based on the mass transfer coefficients obtained in HNO
solution and TDdDGA or HONTA / n-dodecane solvent were evaluated by the single drop technique. Prior to the evaluation of mass transfer coefficient, we had optimized the structure of the single drop apparatus to improve accuracy of the measurement. Based on the mass transfer coefficients obtained in HNO / TDdDGA-n-dodecane system, Ln behaviors in the counter-current extraction and back-extraction using mixer-settlers and centrifugal contactors were estimated by simple calculation, and they had a good agreement with our previous experimental results. We also confirmed the mass transfer coefficients of Ln in HNO
/ TDdDGA-n-dodecane system, Ln behaviors in the counter-current extraction and back-extraction using mixer-settlers and centrifugal contactors were estimated by simple calculation, and they had a good agreement with our previous experimental results. We also confirmed the mass transfer coefficients of Ln in HNO / HONTA - n-dodecane system are under 10
/ HONTA - n-dodecane system are under 10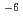 m/s.
m/s.
Shimojo, Kojiro; Suzuki, Hideya; Yokoyama, Keiichi; Yaita, Tsuyoshi; Ikeda, Atsushi
Analytical Sciences, 36(12), p.1435 - 1437, 2020/12
Times Cited Count:13 Percentile:64.65(Chemistry, Analytical)Liquid-liquid extraction for the removal of pertechnetate ( TcO
TcO
 ) and perrhenate (ReO
) and perrhenate (ReO
 ) is reported using tripodal extractant
) is reported using tripodal extractant 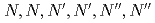 -hexa-
-hexa- -octylnitrilotriacetamide (HONTA) composed of three amide groups and a tertiary amine. The extraction behavior was compared with those using alkyldiamideamines (ADAAM(Oct) and ADAAM(EH)), and the commercial amine-type extractant, trioctylamine (TOA). HONTA quantitatively extracted
-octylnitrilotriacetamide (HONTA) composed of three amide groups and a tertiary amine. The extraction behavior was compared with those using alkyldiamideamines (ADAAM(Oct) and ADAAM(EH)), and the commercial amine-type extractant, trioctylamine (TOA). HONTA quantitatively extracted  TcO
TcO
 and ReO
and ReO
 in the pH range from 1.0 to 2.5 by co-extraction of protons. Extraction performance of extractants was improved in the order of HONTA
in the pH range from 1.0 to 2.5 by co-extraction of protons. Extraction performance of extractants was improved in the order of HONTA  ADAAM(Oct)
ADAAM(Oct)  ADAAM(EH)
ADAAM(EH)  TOA.
TOA.  TcO
TcO
 and ReO
and ReO
 in the extracting phase were successfully stripped using neutral aqueous solutions as the receiving phase, and the extraction ability of HONTA was maintained after five repeated uses.
in the extracting phase were successfully stripped using neutral aqueous solutions as the receiving phase, and the extraction ability of HONTA was maintained after five repeated uses.
Toigawa, Tomohiro; Murayama, Rin*; Kumagai, Yuta; Yamashita, Shinichi*; Suzuki, Hideya; Ban, Yasutoshi; Matsumura, Tatsuro
UTNL-R-0501, p.24 - 25, 2020/12
This report summarizes the results obtained in FY2019 at Electron Linac Facility of University of Tokyo. The radiolysis process of a diglycolamide extractant, which is expected to be used in the separation process of minor actinides (MA), in dodecane and octanol solutions was investigated by pulse radiolysis. As a result, it was suggested that by adding alcohol, the decomposition process of the diglycolamide extractant was different from the decomposition processes in the single solvent of dodecane considered that the decomposition occurred via a radical cation species of the extractant.
Tsutsui, Nao; Ban, Yasutoshi; Suzuki, Hideya*; Nakase, Masahiko*; Ito, Sayumi*; Inaba, Yusuke*; Matsumura, Tatsuro; Takeshita, Kenji*
Analytical Sciences, 36(2), p.241 - 246, 2020/02
Times Cited Count:21 Percentile:79.8(Chemistry, Analytical)To investigate the effective separation of actinides (Ans) from lanthanides (Lns), single-stage batch extraction experiments were performed with a novel extractant, tetradodecyl-1,10-phenanthroline-2,9-diamide (TDdPTDA) with various diluents such as 3-nitrobenzotrifluoride (F-3), nitrobenzene, and  -dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from
-dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from  -dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
-dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (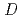
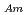 ) and Cm (
) and Cm (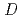
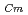 ) for TDdPTDA, with the high separation factors (
) for TDdPTDA, with the high separation factors ( s) of Am from Lns enough for their separation.
s) of Am from Lns enough for their separation.
Tsukahara, Takehiko*; Saga, Kaname*; Suzuki, Hideya*; Matsumura, Tatsuro
Kurin Tekunoroji, 29(12), p.4 - 7, 2019/12
no abstracts in English
 -hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cell
-hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsutsui, Nao; Tsubata, Yasuhiro; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 37(7), p.489 - 499, 2019/11
Times Cited Count:15 Percentile:52.81(Chemistry, Multidisciplinary)A continuous counter-current experiment to separate minor actinides (MAs: Am and Cm) was performed with  -hexaochyl nitrilotriacetamide (HONTA) as an extractant. Nitric acid of 0.08 M (mol/dm
-hexaochyl nitrilotriacetamide (HONTA) as an extractant. Nitric acid of 0.08 M (mol/dm ) containing MAs and rare earths (REs) recovered from high-level waste was used as the Feed, and the experiment was conducted for 14 h. The ratios of Am and Cm recovered into the MA fraction measured 94.9% and 78.9%, respectively. HONTA hardly extracted Y, La, and Eu in the Feed (99.9% for Y, 99.9% for La, and 96.7% for Eu), most of which were distributed to the RE fraction. A portion of Nd was extracted by HONTA, and consequently the ratio of Nd in the RE fraction was 83.5%. The concentrations of MAs and some REs in each stage were calculated using a simulation code, and the results are consistent with the experimental values. This code indicates that the ratios of MAs in the MA fraction and REs in the RE fraction could be
) containing MAs and rare earths (REs) recovered from high-level waste was used as the Feed, and the experiment was conducted for 14 h. The ratios of Am and Cm recovered into the MA fraction measured 94.9% and 78.9%, respectively. HONTA hardly extracted Y, La, and Eu in the Feed (99.9% for Y, 99.9% for La, and 96.7% for Eu), most of which were distributed to the RE fraction. A portion of Nd was extracted by HONTA, and consequently the ratio of Nd in the RE fraction was 83.5%. The concentrations of MAs and some REs in each stage were calculated using a simulation code, and the results are consistent with the experimental values. This code indicates that the ratios of MAs in the MA fraction and REs in the RE fraction could be 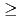 99% by optimizing separation conditions.
99% by optimizing separation conditions.
Morita, Keisuke; Suzuki, Hideya; Matsumura, Tatsuro; Takahashi, Yuya*; Omori, Takashi*; Kaneko, Masaaki*; Asano, Kazuhito*
Proceedings of International Nuclear Fuel Cycle Conference / Light Water Reactor Fuel Performance Conference (Global/Top Fuel 2019) (USB Flash Drive), p.464 - 468, 2019/09
High level liquid waste (HLLW) contains several radionuclides with half-lives longer than 10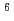 year. For reduce environmental burden of waste disposal, minor actinoids and long-lived fission products will to be partitioned and transmuted. JAEA and Toshiba developed process for recovering Se, Zr, Pd and Cs from HLLW. Solvent extraction for Zr with novel extractant,
year. For reduce environmental burden of waste disposal, minor actinoids and long-lived fission products will to be partitioned and transmuted. JAEA and Toshiba developed process for recovering Se, Zr, Pd and Cs from HLLW. Solvent extraction for Zr with novel extractant,  -didodecyl-2-hydroxyacetoamide (HAA) was detailed. The HAA system showed high selectivity for Zr, as indicated by the extraction order of Zr
-didodecyl-2-hydroxyacetoamide (HAA) was detailed. The HAA system showed high selectivity for Zr, as indicated by the extraction order of Zr  Mo
Mo  Pd
Pd  Ag
Ag 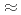 Sb
Sb  Sn
Sn  Lns
Lns  Fe. The extracted species was determined as Zr(HAA)
Fe. The extracted species was determined as Zr(HAA) (NO
(NO )
) (HNO
(HNO )
) . A continuous countercurrent extraction with HAA was applied to a simulated, concentrated HLLW after Pd, Se, and Cs removal, where the quantitative extraction of Zr and Mo was effectively demonstrated.
. A continuous countercurrent extraction with HAA was applied to a simulated, concentrated HLLW after Pd, Se, and Cs removal, where the quantitative extraction of Zr and Mo was effectively demonstrated.
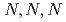 -trimethylglycine
-trimethylglycineSuzuki, Tomoya*; Narita, Hirokazu*; Ogata, Takeshi*; Suzuki, Hideya; Matsumura, Tatsuro; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi
Solvent Extraction Research and Development, Japan, 26(1), p.11 - 19, 2019/06
Times Cited Count:3 Percentile:14.02(Chemistry, Multidisciplinary)The ability of AMP03, a styrene-divinylbenzene copolymer functionalized with 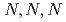 -trimethylglycine moieties, to adsorb Pd(II) from HNO
-trimethylglycine moieties, to adsorb Pd(II) from HNO solutions was investigated to elucidate the affinity of
solutions was investigated to elucidate the affinity of 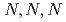 -trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
-trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
 -isopropylacrylamide) with diglycolamide-typed ligands
-isopropylacrylamide) with diglycolamide-typed ligandsSaga, Kaname*; Suzuki, Hideya; Matsumura, Tatsuro; Tsukahara, Takehiko*
Analytical Sciences, 35(4), p.461 - 464, 2019/04
Times Cited Count:3 Percentile:7.76(Chemistry, Analytical)The phase transition-based gelification phenomenon of poly- -isopropylacrylamide (PNIPAAm) in aqueous solutions has a great potential in developing new waste-free extraction processes of metal ions. By using hydrophobic diglycolamide-typed ligands in gelification extraction, a one-step complete extraction of all the RE ions from a nitric acid solution was successfully realized.
-isopropylacrylamide (PNIPAAm) in aqueous solutions has a great potential in developing new waste-free extraction processes of metal ions. By using hydrophobic diglycolamide-typed ligands in gelification extraction, a one-step complete extraction of all the RE ions from a nitric acid solution was successfully realized.
Tsukahara, Takehiko*; Suzuki, Hideya*; Matsumura, Tatsuro; Saga, Kaname*
Bunri Gijutsu, 49(4), p.221 - 225, 2019/04
no abstracts in English
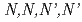 -tetradodecyldiglycolamide (TDdDGA) by using mixer-settler extractors in a hot cell
-tetradodecyldiglycolamide (TDdDGA) by using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya; Hotoku, Shinobu; Kawasaki, Tomohiro*; Sagawa, Hiroshi*; Tsutsui, Nao; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 37(1), p.27 - 37, 2019/00
Times Cited Count:21 Percentile:65.06(Chemistry, Multidisciplinary)A continuous counter-current experiment using TDdDGA was performed using mixer-settler extractors installed in a hot cell. Nitric acid containing minor actinides (MAs: Am and Cm), rare earths (REs: Y, La, Nd, and Eu), and other fission products (Sr, Cs, Zr, Mo, Ru, Rh, and Pd) was fed to the extractor. TDdDGA effectively extracted MAs and REs from the feed, while other fission products were barely extracted. The extracted MAs and REs were back-extracted by bringing them in contact with 0.02 mol/dm nitric acid, and they were collected as the MA-RE fraction. The proportions of MA and RE in the MA-RE fraction were
nitric acid, and they were collected as the MA-RE fraction. The proportions of MA and RE in the MA-RE fraction were  98% and
98% and  86%, respectively. These results demonstrated the applicability of TDdDGA as an extractant for MAs and REs.
86%, respectively. These results demonstrated the applicability of TDdDGA as an extractant for MAs and REs.
Kaneko, Masashi; Suzuki, Hideya; Matsumura, Tatsuro
Inorganic Chemistry, 57(23), p.14513 - 14523, 2018/12
Times Cited Count:20 Percentile:78.15(Chemistry, Inorganic & Nuclear)We elucidated the separation mechanism between Am(III) and Cm(III) ions by using two different types of diamide ligands, diglycolamide (DGA) and alkylated diamide amine (ADAAM), by means of the density functional theory technique and electron density analysis. The molecular geometries and formation reactions of the metal-ligand complexes were modeled by using [M(DGA) ]
] and [M(ADAAM)(NO
and [M(ADAAM)(NO )
) (H
(H O)]. We successfully reproduced Cm(III) selectivity over Am(III) with DGA and Am(III) selectivity over Cm(III) with ADAAM. Furthermore, we analyzed the bonding properties between the metal ion and the diamide-type ligands by using model complexes, [M(DGA)
O)]. We successfully reproduced Cm(III) selectivity over Am(III) with DGA and Am(III) selectivity over Cm(III) with ADAAM. Furthermore, we analyzed the bonding properties between the metal ion and the diamide-type ligands by using model complexes, [M(DGA) ]
] and [M(ADAAM)(NO
and [M(ADAAM)(NO )
) (H
(H O)], and revealed the differences in terms of the bond dissociation energy and the metal 5f-orbital participation in the covalency between the Am(III) and the Cm(III) complexes. It was suggested that the differences were key factors to understand the Am(III)/Cm(III) selectivity.
O)], and revealed the differences in terms of the bond dissociation energy and the metal 5f-orbital participation in the covalency between the Am(III) and the Cm(III) complexes. It was suggested that the differences were key factors to understand the Am(III)/Cm(III) selectivity.
 -P adsorbent for MA(III) recovery
-P adsorbent for MA(III) recoveryKofuji, Hirohide; Watanabe, So; Takeuchi, Masayuki; Suzuki, Hideya; Matsumura, Tatsuro; Shiwaku, Hideaki; Yaita, Tsuyoshi
Progress in Nuclear Science and Technology (Internet), 5, p.61 - 65, 2018/11
Nagano, Tetsushi; Naganawa, Hirochika; Suzuki, Hideya; Toshimitsu, Masaaki*; Mitamura, Hisayoshi*; Yanase, Nobuyuki*; Grambow, B.
Analytical Sciences, 34(9), p.1099 - 1102, 2018/09
Times Cited Count:12 Percentile:45.99(Chemistry, Analytical)A previously reported emulsion flow (EF) extraction system does not include a device for refining used solvent. Therefore, the processing of large quantities of wastewater by using the EF extractor alone could lead to the accumulation of wastewater components into the solvent and diminished extraction performance. In the present study, we have developed a solvent-washing-type EF system, which is equipped with a unit for washing used solvent to prevent accumulation, and successfully applied it for treating uranium-containing wastewater.
Watanabe, So; Suzuki, Hideya; Goto, Ichiro*; Kofuji, Hirohide; Matsumura, Tatsuro
Nihon Ion Kokan Gakkai-Shi, 29(3), p.71 - 75, 2018/09
Suzuki, Hideya; Tsubata, Yasuhiro; Kurosawa, Tatsuya*; Sagawa, Hiroshi*; Matsumura, Tatsuro
Journal of Nuclear Science and Technology, 54(11), p.1163 - 1167, 2017/11
Times Cited Count:26 Percentile:93.3(Nuclear Science & Technology)A highly practical diamide-type extractant, which is an alkyl diamide amine with 2-ethylhexyl alkyl chains (ADAAM(EH)), was investigated for mutual separation of Am(III) and Cm(III). ADAAM(EH) is a multidentate ligand with one soft N-donor atom and two hard O-donor atoms in its central frame. This tridentate arrangement of donor atoms provides selective binding to Am(III) compared to that with Cm(III) in highly acidic media, resulting in separation factors of up to 5.5. A continuous liquid-liquid extraction and stripping test was conducted using a multistage countercurrent mixer-settler extractor with ADAAM(EH) in n-dodecane. In this test, separation of Am(III) and Cm(III) was achieved with very high yield.