Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yamaguchi, Akiko; Kurihara, Yuichi*; Nagata, Kojiro*; Tanaka, Kazuya; Higaki, Shogo*; Kobayashi, Toru; Tanida, Hajime; Ohara, Yoshiyuki*; Yokoyama, Keiichi; Yaita, Tsuyoshi; et al.
Journal of Colloid and Interface Science, 661, p.317 - 332, 2024/05
Times Cited Count:0no abstracts in English
Yamaguchi, Akiko; Okumura, Masahiko; Takahashi, Yoshio*
Isotope News, (789), p.20 - 23, 2023/10
Radium is a radioactive element produced from uranium and thorium and is important for environmental contamination issues around uranium mines and for geological disposal. In addition, radium is used in radiometric dating and cancer therapy, making it important not only in environmental chemistry but also in many other fields, including geochemistry and nuclear medicine. However, because radium is a radioactive element with no stable isotopes, spectroscopic measurement of radium is difficult, and little information at the molecular level has been obtained so far. In this study, we have clarified the molecular-level information of hydrated radium for the first time in the world by combining extended X-ray absorption fine structure (EXAFS) measurements and first-principles molecular dynamics simulations.
 -edge for Ce in bauxites using transition-edge sensors; Implications for Ti-rich geological samples
-edge for Ce in bauxites using transition-edge sensors; Implications for Ti-rich geological samplesLi, W.*; Yamada, Shinya*; Hashimoto, Tadashi; Okumura, Takuma*; Hayakawa, Ryota*; Nitta, Kiyofumi*; Sekizawa, Oki*; Suga, Hiroki*; Uruga, Tomoya*; Ichinohe, Yuto*; et al.
Analytica Chimica Acta, 1240, p.340755_1 - 340755_9, 2023/02
Times Cited Count:2 Percentile:31.9(Chemistry, Analytical)no abstracts in English

 -, SeO
-, SeO
 -, and SeO
-, and SeO
 -coprecipitated barite after treatment with phosphate
-coprecipitated barite after treatment with phosphateTokunaga, Kohei; Tanaka, Kazuya; Takahashi, Yoshio*; Kozai, Naofumi
Environmental Science & Technology, 57(8), p.3166 - 3175, 2023/02
Times Cited Count:1 Percentile:52.26(Engineering, Environmental)Coprecipitation of radionuclides with barite has been studied to remove radionuclides from radioactive liquid waste because of its excellent removal efficiency; however, little information exists concerning the stability of the ions coprecipitated with barite. This study systematically investigated the stability of iodate, selenite, and selenate coprecipitated with barite via leaching tests. These oxyanions were gradually leached from the oxyanion-bearing barite into ultrapure water over time. Leaching of the oxyanions significantly increased in leaching solutions containing NaCl (pH5.3), NaNO (pH5.9), and Na
(pH5.9), and Na SO
SO (pH5.7). Conversely, leaching of the oxyanions was suppressed in KH
(pH5.7). Conversely, leaching of the oxyanions was suppressed in KH PO
PO solution (pH8.5), indicating that phosphate stabilized the oxyanion-bearing barite. The effect of phosphate treatment on oxyanion-bearing barite was further investigated. The results showed that the barite surface was modified with phosphate, and a thin surface layer of a barium phosphate-like structure was formed. The amount of oxyanions leached from the phosphate-treated samples into leaching solutions containing NaCl or NaNO
solution (pH8.5), indicating that phosphate stabilized the oxyanion-bearing barite. The effect of phosphate treatment on oxyanion-bearing barite was further investigated. The results showed that the barite surface was modified with phosphate, and a thin surface layer of a barium phosphate-like structure was formed. The amount of oxyanions leached from the phosphate-treated samples into leaching solutions containing NaCl or NaNO was much lower than the amounts leached from the untreated barite samples into ultrapure water. The barite coprecipitation combined with subsequent phosphate treatment may be a promising method to efficiently remove iodate, selenite, and selenate from wastewater and stabilize them as barite coprecipitates.
was much lower than the amounts leached from the untreated barite samples into ultrapure water. The barite coprecipitation combined with subsequent phosphate treatment may be a promising method to efficiently remove iodate, selenite, and selenate from wastewater and stabilize them as barite coprecipitates.
Tokunaga, Kohei; Takahashi, Yoshio*; Kozai, Naofumi
Journal of Nuclear and Radiochemical Sciences (Internet), 23, p.14 - 19, 2023/00
 -edge to assess the U(V) electronic structure in FeUO
-edge to assess the U(V) electronic structure in FeUO
Yomogida, Takumi; Akiyama, Daisuke*; Ouchi, Kazuki; Kumagai, Yuta; Higashi, Kotaro*; Kitatsuji, Yoshihiro; Kirishima, Akira*; Kawamura, Naomi*; Takahashi, Yoshio*
Inorganic Chemistry, 61(50), p.20206 - 20210, 2022/12
Times Cited Count:2 Percentile:36.89(Chemistry, Inorganic & Nuclear)FeUO was studied to clarify the electronic structure of U(V) in a metal monouranate compound. We obtained the peak splitting of HERFD-XANES spectra utilizing high-energy-resolution fluorescence detection-X-ray absorption near edge structure (HERFD-XANES) spectroscopy at the U L
was studied to clarify the electronic structure of U(V) in a metal monouranate compound. We obtained the peak splitting of HERFD-XANES spectra utilizing high-energy-resolution fluorescence detection-X-ray absorption near edge structure (HERFD-XANES) spectroscopy at the U L -edge, which is a novel technique in the U(V) compounds. Theoretical calculations revealed that the peak splitting was caused by splitting the 6d orbital of U(V). Such distinctive electronic states are of major interest to researchers and engineers working in various fields, from fundamental physics to the nuclear industry and environmental sciences for actinide elements.
-edge, which is a novel technique in the U(V) compounds. Theoretical calculations revealed that the peak splitting was caused by splitting the 6d orbital of U(V). Such distinctive electronic states are of major interest to researchers and engineers working in various fields, from fundamental physics to the nuclear industry and environmental sciences for actinide elements.
 molecular dynamics simulations reveal the hydration structure of the radium(II) ion
molecular dynamics simulations reveal the hydration structure of the radium(II) ionYamaguchi, Akiko; Nagata, Kojiro*; Kobayashi, Keita; Tanaka, Kazuya; Kobayashi, Toru; Tanida, Hajime; Shimojo, Kojiro; Sekiguchi, Tetsuhiro; Kaneta, Yui; Matsuda, Shohei; et al.
iScience (Internet), 25(8), p.104763_1 - 104763_12, 2022/08
Times Cited Count:9 Percentile:68.46(Multidisciplinary Sciences)no abstracts in English
Shiraishi, Fumito*; Chihara, Ryoji*; Tanimoto, Risa*; Tanaka, Kazuya; Takahashi, Yoshio*
Island Arc, 31(1), p.e12448_1 - e12448_9, 2022/05
Times Cited Count:0 Percentile:0(Geosciences, Multidisciplinary)At Yunotaki Fall in north Japan, manganese-oxidizing bacteria were previously assumed to have oxidized manganese to precipitate birnessite, which relied on oxygen released from algae. However, it remained unclear whether larger-scale manganese oxide precipitation was actually occurring under light conditions. This study evaluated the contribution of indirect oxidation using microelectrodes to analyze local water chemistry, in addition to bulk water chemistry and DNA analyses. The results of this study demonstrate that low bulk pH values in the hot spring water hindered indirect oxidation despite the occurrence of active oxygenic photosynthesis and that direct oxidation by manganese-oxidizing bacteria is considered to dominate in the investigated sample.
 Cs activity concentration in the aquatic insect
Cs activity concentration in the aquatic insect  (Tricoptera: Stenopsychidae) in the Ota River, Fukushima, Japan
(Tricoptera: Stenopsychidae) in the Ota River, Fukushima, JapanIshii, Yumiko*; Miura, Hikaru*; Jo, J.*; Tsuji, Hideki*; Saito, Rie; Koarai, Kazuma; Hagiwara, Hiroki; Urushidate, Tadayuki*; Nishikiori, Tatsuhiro*; Wada, Toshihiro*; et al.
PLOS ONE (Internet), 17(5), p.e0268629_1 - e0268629_17, 2022/05
Times Cited Count:3 Percentile:43.07(Multidisciplinary Sciences)We investigated the variability in  Cs activity concentration in individual aquatic insects in detritivorous caddisfly (
Cs activity concentration in individual aquatic insects in detritivorous caddisfly ( ) and carnivorous dobsonfly (
) and carnivorous dobsonfly ( ) larvae from the Ota River, Fukushima. Caddisfly larvae showed sporadically higher radioactivity, whereas no such outliers were observed in dobsonfly larvae. Autoradiography and scanning electron microscopy analyses confirmed that these caddisfly larvae samples contained radiocesium-bearing microparticles (CsMPs), which are insoluble Cs-bearing silicate glass particles. CsMPs were also found in potential food sources of caddisfly larvae, such as periphyton and drifting particulate organic matter, indicating that larvae may ingest CsMPs along with food particles of similar size. Although CsMPs distribution and uptake by organisms in freshwater ecosystems is relatively unknown, our study demonstrates that CsMPs can be taken up by aquatic insects.
) larvae from the Ota River, Fukushima. Caddisfly larvae showed sporadically higher radioactivity, whereas no such outliers were observed in dobsonfly larvae. Autoradiography and scanning electron microscopy analyses confirmed that these caddisfly larvae samples contained radiocesium-bearing microparticles (CsMPs), which are insoluble Cs-bearing silicate glass particles. CsMPs were also found in potential food sources of caddisfly larvae, such as periphyton and drifting particulate organic matter, indicating that larvae may ingest CsMPs along with food particles of similar size. Although CsMPs distribution and uptake by organisms in freshwater ecosystems is relatively unknown, our study demonstrates that CsMPs can be taken up by aquatic insects.
Yamaguchi, Akiko; Nagata, Kojiro*; Tanaka, Kazuya; Kobayashi, Keita; Kobayashi, Toru; Shimojo, Kojiro; Tanida, Hajime; Sekiguchi, Tetsuhiro; Kaneta, Yui; Matsuda, Shohei; et al.
Hosha Kagaku, (45), p.28 - 30, 2022/03
no abstracts in English
Miura, Hikaru*; Kuribara, Yuichi; Takahashi, Yoshio*
Chikyu Kagaku, 55(4), p.122 - 131, 2021/12
Radiocesium-bearing microparticles (CsMPs), glassy water-resistant particles with highly concentrated radiocesium, were emitted by the Fukushima Daiichi Nuclear Power Plant accident. Since first discovery of CsMPs, a number of studies have analyzed the particles isolated from environmental samples and revealed their physical and chemical properties, distribution, and migration. This paper is intended to provide an overview focusing on the environmental transport and impact of CsMPs. First, we begin by reviewing the relationship between deposition areas and atmospheric plumes of CsMPs found on land. Next, search and separation methods for CsMPs will be described. Then, secondary transport via rivers and effect of CsMPs on Kd values of Cs in rivers will be discussed. Finally, CsMPs found in the ocean and their difference from terrestrial ones will be summarized.
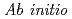 molecular dynamics simulations using the SCAN meta-GGA density functional and EXAFS spectroscopy studies
molecular dynamics simulations using the SCAN meta-GGA density functional and EXAFS spectroscopy studiesYamaguchi, Akiko; Kobayashi, Keita; Takahashi, Yoshio*; Machida, Masahiko; Okumura, Masahiko
Chemical Physics Letters, 780, p.138945_1 - 138945_5, 2021/10
Times Cited Count:5 Percentile:44.89(Chemistry, Physical)no abstracts in English
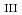 -edge for the determination of oxidation state for trace amount of Eu in natural samples by bragg-type crystal analyzer system
-edge for the determination of oxidation state for trace amount of Eu in natural samples by bragg-type crystal analyzer systemKonagaya, Rimi*; Kawamura, Naomi*; Yamaguchi, Akiko; Takahashi, Yoshio*
Chemistry Letters, 50(8), p.1570 - 1572, 2021/08
Times Cited Count:3 Percentile:22.95(Chemistry, Multidisciplinary)no abstracts in English
Tokunaga, Kohei; Takahashi, Yoshio*; Tanaka, Kazuya; Kozai, Naofumi
Chemosphere, 266, p.129104_1 - 129104_10, 2021/03
Times Cited Count:10 Percentile:52.13(Environmental Sciences)Radioactive iodine (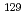 I) is of great concern owing to its high mobility in the environment and long-term radiotoxicity, but there is a lack of effective techniques for removing iodate (IO
I) is of great concern owing to its high mobility in the environment and long-term radiotoxicity, but there is a lack of effective techniques for removing iodate (IO
 ) from aqueous solution. The aim of this study is to develop a new technique for removing radioactive iodate from contaminated solution by using barite (BaSO
) from aqueous solution. The aim of this study is to develop a new technique for removing radioactive iodate from contaminated solution by using barite (BaSO ). In the present study, we examined the coprecipitation mechanism of iodate by barite at the molecular level for determining optimum conditions for iodate removal. The results showed that iodate was effectively removed from aqueous solution by coprecipitation, even in the presence of competitive anions in solution. Comparing our method with previous studies, iodate removal efficiency by barite was determined to be about two orders of magnitude greater than that by hydrotalcite-like layered double hydroxide at Cl
). In the present study, we examined the coprecipitation mechanism of iodate by barite at the molecular level for determining optimum conditions for iodate removal. The results showed that iodate was effectively removed from aqueous solution by coprecipitation, even in the presence of competitive anions in solution. Comparing our method with previous studies, iodate removal efficiency by barite was determined to be about two orders of magnitude greater than that by hydrotalcite-like layered double hydroxide at Cl concentration of 10 mmol L
concentration of 10 mmol L . Extended X-ray absorption fine structure (EXAFS) analysis indicated that incorporated iodate was strongly bound in the crystal lattice of barite by substituting the sulfate site in the structure when the iodine concentration was low. The charge compensation problem from the IO
. Extended X-ray absorption fine structure (EXAFS) analysis indicated that incorporated iodate was strongly bound in the crystal lattice of barite by substituting the sulfate site in the structure when the iodine concentration was low. The charge compensation problem from the IO
 substitution in SO
substitution in SO
 site is achieved by the substitution of Na
site is achieved by the substitution of Na -IO
-IO
 pairs at the nearest Ba
pairs at the nearest Ba site. Therefore, considering high removal efficiency and strong binding of iodate in barite, coprecipitation with barite is a promising material for removing radioactive iodate from various aqueous solutions contaminated with iodate.
site. Therefore, considering high removal efficiency and strong binding of iodate in barite, coprecipitation with barite is a promising material for removing radioactive iodate from various aqueous solutions contaminated with iodate.
Miyajima, Yusuke*; Saito, Ayaka*; Kagi, Hiroyuki*; Yokoyama, Tatsunori; Takahashi, Yoshio*; Hirata, Takafumi*
Geostandards and Geoanalytical Research, 45(1), p.189 - 205, 2021/03
Times Cited Count:5 Percentile:21.77(Geochemistry & Geophysics)Uncertainty for elemental and isotopic analyses of calcite by LA-ICP-MS is largely controlled by the homogeneity of the reference materials (RMs) used for normalization and validation. In order to produce calcite RMs with homogeneous elemental and isotopic compositions, we incorporated elements including U, Pb, and rare earth elements into calcite through heat- and pressure-induced crystallization from amorphous calcium carbonate that was precipitated from element-doped reagent solution. X-ray absorption spectra showed that U was present as U(VI) in the synthesized calcite, probably with a different local structure from that of aqueous uranyl ions. The uptake rate of U by our calcite was higher in comparison to synthetic calcite of previous studies. Variations of element mass fractions in the calcite were better than 12% 2RSD, mostly within 7%. The 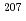 Pb/
Pb/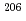 Pb ratio in the calcite showed
Pb ratio in the calcite showed  1% variations, while the
1% variations, while the 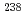 U/
U/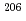 Pb ratio showed 3-24% variations depending on element mass fractions. Using the synthetic calcite as primary RMs, we could date a natural calcite RM, WC-1, with analytical uncertainty as low as
Pb ratio showed 3-24% variations depending on element mass fractions. Using the synthetic calcite as primary RMs, we could date a natural calcite RM, WC-1, with analytical uncertainty as low as  3%. The method presented can be useful to produce calcite with controlled and homogeneous element mass fractions, and is a promising alternative to natural calcite RMs for U-Pb geochronology.
3%. The method presented can be useful to produce calcite with controlled and homogeneous element mass fractions, and is a promising alternative to natural calcite RMs for U-Pb geochronology.
Miura, Hikaru*; Ishimaru, Takashi*; Ito, Yukari*; Kuribara, Yuichi; Otosaka, Shigeyoshi*; Sakaguchi, Aya*; Misumi, Kazuhiro*; Tsumune, Daisuke*; Kubo, Atsushi*; Higaki, Shogo*; et al.
Scientific Reports (Internet), 11, p.5664_1 - 5664_11, 2021/03
Times Cited Count:13 Percentile:67.27(Multidisciplinary Sciences)For the first time, we isolated and investigated seven CsMPs (radioactive caesium-bearing microparticles) from marine particulate matter and sediment. From the elemental composition, the 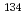 Cs/
Cs/ Cs activity ratio, and the
Cs activity ratio, and the  Cs activity per unit volume results, we inferred that the five CsMPs collected from particulate matter were emitted from Unit 2 of the FDNPP, whereas the two CsMPs collected from marine sediment were possibly emitted from Unit 3. The presence of CsMPs can cause overestimation of the solid-water distribution coefficient of Cs in marine sediments and particulate matter and a high apparent radiocaesium concentration factor for marine biota. CsMPs emitted from Unit 2, which were collected from the estuary of a river that flowed through a highly contaminated area, may have been deposited on land and then transported by the river. By contrast, CsMPs emitted from Unit 3 were possibly transported eastward by the wind and deposited directly onto the ocean surface.
Cs activity per unit volume results, we inferred that the five CsMPs collected from particulate matter were emitted from Unit 2 of the FDNPP, whereas the two CsMPs collected from marine sediment were possibly emitted from Unit 3. The presence of CsMPs can cause overestimation of the solid-water distribution coefficient of Cs in marine sediments and particulate matter and a high apparent radiocaesium concentration factor for marine biota. CsMPs emitted from Unit 2, which were collected from the estuary of a river that flowed through a highly contaminated area, may have been deposited on land and then transported by the river. By contrast, CsMPs emitted from Unit 3 were possibly transported eastward by the wind and deposited directly onto the ocean surface.
Tanaka, Kazuya; Kanasashi, Tsutomu*; Takenaka, Chisato*; Takahashi, Yoshio*
Science of the Total Environment, 755(Part 2), p.142598_1 - 142598_8, 2021/02
Times Cited Count:5 Percentile:35.21(Environmental Sciences)In this study, we investigated coordination structures of Cs in 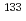 Cs-doped bark, sapwood, heartwood, needle, and branch samples of trees collected in Fukushima by extended X-ray absorption fine structure (EXAFS) spectroscopy. We examined four representative tree species in Fukushima,
Cs-doped bark, sapwood, heartwood, needle, and branch samples of trees collected in Fukushima by extended X-ray absorption fine structure (EXAFS) spectroscopy. We examined four representative tree species in Fukushima, 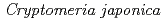 ,
, 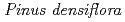 ,
, 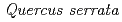 , and
, and 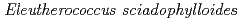 . EXAFS spectra suggested that Cs was adsorbed as an outer-sphere complex on all parts of the four species, with electrostatic binding to negatively charged functional groups in components of tree tissues. These results were supported by extraction experiments where most of the sorbed Cs was desorbed from all parts of each tree species using 1 M CH
. EXAFS spectra suggested that Cs was adsorbed as an outer-sphere complex on all parts of the four species, with electrostatic binding to negatively charged functional groups in components of tree tissues. These results were supported by extraction experiments where most of the sorbed Cs was desorbed from all parts of each tree species using 1 M CH COONH
COONH .
.
Nagasawa, Makoto*; Qin, H.-B.*; Yamaguchi, Akiko; Takahashi, Yoshio*
Chemistry Letters, 49(8), p.909 - 911, 2020/08
Times Cited Count:3 Percentile:10.1(Chemistry, Multidisciplinary)Miura, Hikaru*; Kuribara, Yuichi; Yamamoto, Masayoshi*; Sakaguchi, Aya*; Yamaguchi, Noriko*; Sekizawa, Oki*; Nitta, Kiyofumi*; Higaki, Shogo*; Tsumune, Daisuke*; Itai, Takaaki*; et al.
Scientific Reports (Internet), 10, p.11421_1 - 11421_9, 2020/07
Times Cited Count:18 Percentile:68.95(Multidisciplinary Sciences)Sugiura, Yuki; Tomura, Tsutomu*; Ishidera, Takamitsu; Doi, Reisuke; Francisco, P. C. M.; Shiwaku, Hideaki; Kobayashi, Toru; Matsumura, Daiju; Takahashi, Yoshio*; Tachi, Yukio
Journal of Radioanalytical and Nuclear Chemistry, 324(2), p.615 - 622, 2020/05
Times Cited Count:4 Percentile:45.45(Chemistry, Analytical)