Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hoshina, Hiroyuki; Kasai, Noboru; Amada, Haruyo; Takahashi, Makikatsu*; Tanaka, Kazuya*; Seko, Noriaki
Nihon Ion Kokan Gakkai-Shi, 25(4), p.248 - 251, 2014/11
Amada, Haruyo; Takahashi, Makikatsu*; Hoshina, Hiroyuki; Seko, Noriaki
Nihon Ion Kokan Gakkai-Shi, 25(4), p.109 - 113, 2014/11
Hoshina, Hiroyuki; Takahashi, Makikatsu*; Kasai, Noboru; Seko, Noriaki
International Journal of Organic Chemistry, 2(3), p.173 - 177, 2012/09
Hoshina, Hiroyuki; Kasai, Noboru; Shibata, Takuya*; Aketagawa, Yasushi*; Takahashi, Makikatsu*; Yoshii, Akihiro*; Tsunoda, Yasuhiko*; Seko, Noriaki
Radiation Physics and Chemistry, 81(8), p.1033 - 1035, 2012/08
Times Cited Count:4 Percentile:31.8(Chemistry, Physical)Kasai, Noboru; Hoshina, Hiroyuki; Seko, Noriaki; Shibata, Takuya*; Takahashi, Makikatsu*
JAEA-Review 2011-043, JAEA Takasaki Annual Report 2010, P. 46, 2012/01
no abstracts in English
Seko, Noriaki; Hoshina, Hiroyuki; Kasai, Noboru; Ueki, Yuji; Tamada, Masao; Kiryu, Toshiyuki*; Tanaka, Kazuya*; Takahashi, Makikatsu*
Nihon Ion Kokan Gakkai-Shi, 21(3), p.117 - 122, 2010/07

 ions
ionsTakahashi, Yasuyuki; Narumi, Kazumasa; Chiba, Atsuya; Saito, Yuichi; Yamada, Keisuke; Ishikawa, Norito; Sugai, Hiroyuki; Maeda, Yoshihito
EPL; A Letters Journal Exploring the Frontiers of Physics, 88(6), p.63001_1 - 63001_6, 2009/12
Times Cited Count:7 Percentile:45.45(Physics, Multidisciplinary)C
 and C
and C
 ions with 62.5-250 keV/u were incident on self-supporting amorphous carbon foils of 1.4-150
ions with 62.5-250 keV/u were incident on self-supporting amorphous carbon foils of 1.4-150  g/cm
g/cm (70-7500
(70-7500  ). The secondary electrons emitted in the forward direction from a carbon foil were detected by a microchannel-plate detector placed at the exit side of the target. The vicinage effect on the secondary-electron yield was evaluated with the ratio of the secondary-electron yield R
). The secondary electrons emitted in the forward direction from a carbon foil were detected by a microchannel-plate detector placed at the exit side of the target. The vicinage effect on the secondary-electron yield was evaluated with the ratio of the secondary-electron yield R =
= 
 /2
/2
 , where
, where 
 and
and 
 are the yields induced by the C
are the yields induced by the C
 and C
and C
 ion with the same velocity, respectively. For the first time the disappearance of the vicinage effect on the secondary-electron yield from amorphous carbon foils bombarded with 62.5 keV/u C
ion with the same velocity, respectively. For the first time the disappearance of the vicinage effect on the secondary-electron yield from amorphous carbon foils bombarded with 62.5 keV/u C
 ions was observed for thick foils of 61-150
ions was observed for thick foils of 61-150  g/cm
g/cm . The internuclear distance between the fragment ions at the exit of the target was evaluated by calculating trajectories of the fragment ions considering the Coulomb explosion. For a 62.5 keV/u C
. The internuclear distance between the fragment ions at the exit of the target was evaluated by calculating trajectories of the fragment ions considering the Coulomb explosion. For a 62.5 keV/u C
 ion, we have determined the threshold internuclear distance where the vicinage effect disappears exits between 6
ion, we have determined the threshold internuclear distance where the vicinage effect disappears exits between 6  and 23
and 23  . It is expected that the vicinage effect on the energy loss (production process) in this velocity region disappears at the internuclear distance of a few
. It is expected that the vicinage effect on the energy loss (production process) in this velocity region disappears at the internuclear distance of a few  . This result means that the transport or transmission process is important for the appearance of the vicinage effect. Moreover, the threshold internuclear distance depends on the velocity of the ion and increases as the velocity increases. The average charge of the ion increases with increase of the velocity of the ion. These mean that there is a possibility that a charge state plays an important role in the origin of the vicinage effect. In order to account for the experimental results, we discussed two models taking account of two kinds of potentials induced in response to the charge of the fragment ion in the transport process.
. This result means that the transport or transmission process is important for the appearance of the vicinage effect. Moreover, the threshold internuclear distance depends on the velocity of the ion and increases as the velocity increases. The average charge of the ion increases with increase of the velocity of the ion. These mean that there is a possibility that a charge state plays an important role in the origin of the vicinage effect. In order to account for the experimental results, we discussed two models taking account of two kinds of potentials induced in response to the charge of the fragment ion in the transport process.
 value of hydroxyl radical at high temperatures
value of hydroxyl radical at high temperaturesMuroya, Yusa*; Takahashi, Hiroyuki*; Katsumura, Yosuke; Lin, M.; Kumagai, Yuta; Kudo, Hisaaki*
Proceedings of Symposium on Water Chemistry and Corrosion in Nuclear Power Plants in Asia 2009, p.71 - 75, 2009/10
Hydroxyl radical is one of the most important water decomposition products because it has strong oxidative property. However, its property higher than 200 C has not yet been clarified well. In this work, reaction kinetics and pK
C has not yet been clarified well. In this work, reaction kinetics and pK value of OH radical at elevated temperature over 300
value of OH radical at elevated temperature over 300 C have been investigated by means of nanosecond pulse radiolysis. Transient absorption spectra of formed radical and rate constants for the reaction with some solutes in aqueous solutions of benzoate, nitrobenzene, and carbonate etc., have been investigated. In addition, temperature dependent pK
C have been investigated by means of nanosecond pulse radiolysis. Transient absorption spectra of formed radical and rate constants for the reaction with some solutes in aqueous solutions of benzoate, nitrobenzene, and carbonate etc., have been investigated. In addition, temperature dependent pK value (
value ( OH/O
OH/O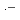 ) up to 300
) up to 300 C has also been evaluated by direct trace and indirect competitive reaction method.
C has also been evaluated by direct trace and indirect competitive reaction method.
Nakahira, Masataka; *; *; Koizumi, Koichi
J. Robot. Mechatron., 10(2), p.116 - 120, 1998/00
no abstracts in English
Takahashi, Hiroyuki*; Katsumura, Yosuke; Lin, M.; Muroya, Yusa*; Kumagai, Yuta
no journal, ,
no abstracts in English

Lin, M.; Katsumura, Yosuke; Muroya, Yusa*; Han, Z.*; Takahashi, Hiroyuki*
no journal, ,
G values of water decomposition products have been investigated up to 500 C by pulse radiolysis techniques. Very significant temperature and pressure effects on G values have been observed around the critical point (Tc=374
C by pulse radiolysis techniques. Very significant temperature and pressure effects on G values have been observed around the critical point (Tc=374 C, Pc=22.1 MPa), while the pressure effect becomes less important as temperature increases and almost disappears at 500
C, Pc=22.1 MPa), while the pressure effect becomes less important as temperature increases and almost disappears at 500 C.
C.
Takahashi, Hiroyuki*; Katsumura, Yosuke; Lin, M.; Muroya, Yusa*; Kumagai, Yuta; Kudo, Hisaaki*
no journal, ,
Using pulse radiolysis techniques, the transient species produced by the reaction of benzoic acid and the transients of water radiolysis were identified and the rate constants with OH radical at elevated temperatures was measured. In addition, we've tried to determine the pKa of OH radical in elevated temperature water based on the rate constants.
Takahashi, Hiroyuki*; Katsumura, Yosuke; Lin, M.; Muroya, Yusa*; Kumagai, Yuta; Kudo, Hisaaki*
no journal, ,
We have investigated radiation chemical reactions in benzoate aqueous aolution by pulse radiolysis. The transient absorption spectra, the rate constant for the reaction of benzoate ion with the hydroxyl radical, and the acid dissociation constant of the hydroxyl radical have been determined. In addition, temperature dependent pK (
( OH/O
OH/O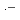 ) value has also been evaluated.
) value has also been evaluated.
Takahashi, Hiroyuki*; Katsumura, Yosuke; Lin, M.; Muroya, Yusa*; Kumagai, Yuta; Kudo, Hisaaki*
no journal, ,
Takahashi, Hiroyuki*; Katsumura, Yosuke; Lin, M.; Muroya, Yusa*; Kumagai, Yuta; Kudo, Hisaaki*
no journal, ,
no abstracts in English
Lin, M.; Katsumura, Yosuke; Muroya, Yusa*; Fu, H.*; Takahashi, Hiroyuki*
no journal, ,
This study is one in a series work aiming at the estimation of the radiolytic yields of water decomposition products in elevated temperatures and supercritical water. OH radical could be the most important oxidizing species that is assumed to be closely related to the corrosion of the structural materials in nuclear reactors. The studies of OH radical at elevated temperatures or supercritical water are thus essential.
Takahashi, Hiroyuki*; Katsumura, Yosuke; Lin, M.; Muroya, Yusa*; Kumagai, Yuta; Kudo, Hisaaki*
no journal, ,
Water radiolysis under HPHT (high-temperature and high-pressure) conditions is of crucial importance in management of water coolant in water-cooled nuclear reactors. This work aims to measure and estimate  of an acido-basic equlibrium of hydroxyl radical (
of an acido-basic equlibrium of hydroxyl radical ( OH),
OH),  OH
OH 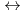 H
H + O
+ O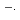 , up to temperatures as high as used in actual operations. Benzoate anion, one of typical aromatic compounds, was used for scavenger toward
, up to temperatures as high as used in actual operations. Benzoate anion, one of typical aromatic compounds, was used for scavenger toward  OH and O
OH and O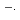 . Pulse radiolysis technique was applied to obtain transient absorption spectra of transient species produced in the scavenging reactions as well as to determine rate constants of the reactions from build-up kinetics and competition method. In addition, it was also intended to estimate
. Pulse radiolysis technique was applied to obtain transient absorption spectra of transient species produced in the scavenging reactions as well as to determine rate constants of the reactions from build-up kinetics and competition method. In addition, it was also intended to estimate 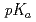 of
of  OH at elevated temperature based on the measured rate constants.
OH at elevated temperature based on the measured rate constants.
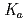 of
of  OH radical
OH radicalTakahashi, Hiroyuki*; Katsumura, Yosuke; Lin, M.; Muroya, Yusa*; Kumagai, Yuta; Kudo, Hisaaki*
no journal, ,
Pulse radiolysis technique was applied to investigate radiation chemical reactions in benzoate aqueous solution. First, transient absorption spectra were observed for visible region to identify transient species produced in the scavenging reactions of benzoate anion with  OH as well as with O
OH as well as with O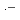 . Subsequently, rate constants of the scavenging reactions were determined from build-up kinetics of the transient species as well as from competition method. In addition, we tried to estimate acid dissociation constant, p
. Subsequently, rate constants of the scavenging reactions were determined from build-up kinetics of the transient species as well as from competition method. In addition, we tried to estimate acid dissociation constant, p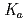 , of
, of  OH at high temperatures based on the rate constants we determined.
OH at high temperatures based on the rate constants we determined.
Takahashi, Hiroyuki*; Katsumura, Yosuke; Lin, M.; Muroya, Yusa*; Kumagai, Yuta; Kudo, Hisaaki*
no journal, ,
It was purposed in this work to determine acid dissociation constant of hydroxyl radical ( OH) at elevated temperatures because the acido-basic equilibrium reaction,
OH) at elevated temperatures because the acido-basic equilibrium reaction,  OH
OH 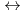 H
H + O
+ O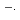 , is one of the most important reactions in water radiolysis and such information is of crucial importance in management of water coolant in water-cooled nuclear reactors. Benzoic acid, one of typical aromatic compounds, was used as scavenges not only toward
, is one of the most important reactions in water radiolysis and such information is of crucial importance in management of water coolant in water-cooled nuclear reactors. Benzoic acid, one of typical aromatic compounds, was used as scavenges not only toward  OH but also toward O
OH but also toward O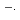 . Pulse radiolysis technique was applied to determine rate constants of the scavenging reactions as well as to observe transient spectra of products in the scavenging reactions. In addition, it was also tried to estimate the p
. Pulse radiolysis technique was applied to determine rate constants of the scavenging reactions as well as to observe transient spectra of products in the scavenging reactions. In addition, it was also tried to estimate the p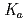 value at elevated temperatures.
value at elevated temperatures.
Seko, Noriaki; Hoshina, Hiroyuki; Kasai, Noboru; Tamada, Masao; Kiryu, Toshiyuki*; Yamaguchi, Takashi*; Yamamoto, Hideo*; Tanaka, Kazuya*; Anzai, Yasuhiro*; Anzai, Nobuhiro*; et al.
no journal, ,
no abstracts in English