Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
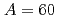
Tamii, Atsushi*; Pellegri, L.*; S derstr
derstr m, P.-A.*; Allard, D.*; Goriely, S.*; Inakura, Tsunenori*; Khan, E.*; Kido, Eiji*; Kimura, Masaaki*; Litvinova, E.*; et al.
m, P.-A.*; Allard, D.*; Goriely, S.*; Inakura, Tsunenori*; Khan, E.*; Kido, Eiji*; Kimura, Masaaki*; Litvinova, E.*; et al.
European Physical Journal A, 59(9), p.208_1 - 208_21, 2023/09
Times Cited Count:1 Percentile:0.02(Physics, Nuclear)no abstracts in English
Oba, Yojiro; Adachi, Nozomu*; Todaka, Yoshikazu*; Gilbert, E. P.*; Mamiya, Hiroaki*
Physical Review Research (Internet), 2(3), p.033473_1 - 033473_6, 2020/11
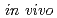 dosimetry system based on an optical fiber-coupled microsized photostimulable phosphor for stereotactic body radiation therapy
dosimetry system based on an optical fiber-coupled microsized photostimulable phosphor for stereotactic body radiation therapyYada, Ryuichi*; Maenaka, Kazusuke*; Miyamoto, Shuji*; Okada, Go*; Sasakura, Aki*; Ashida, Motoi*; Adachi, Masashi*; Sato, Tatsuhiko; Wang, T.*; Akasaka, Hiroaki*; et al.
Medical Physics, 47(10), p.5235 - 5249, 2020/10
Times Cited Count:7 Percentile:52.3(Radiology, Nuclear Medicine & Medical Imaging)The 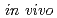 dosimeter system is capable of real-time, accurate, and precise measurement under stereotactic body radiation therapy (SBRT) conditions. The probe is smaller than a conventional dosimeter, has excellent spatial resolution, and can be valuable in SBRT with a steep dose distribution over a small field. The developed PSP dosimeter system appears to be suitable for in vivo SBRT dosimetry.
dosimeter system is capable of real-time, accurate, and precise measurement under stereotactic body radiation therapy (SBRT) conditions. The probe is smaller than a conventional dosimeter, has excellent spatial resolution, and can be valuable in SBRT with a steep dose distribution over a small field. The developed PSP dosimeter system appears to be suitable for in vivo SBRT dosimetry.
 O(
O( He,
He, )
) F reaction; Identification of the low-energy "super" -Gamow-Teller state
F reaction; Identification of the low-energy "super" -Gamow-Teller stateFujita, Hirohiko*; Fujita, Yoshitaka*; Utsuno, Yutaka; Yoshida, Kenichi*; Adachi, Tatsuya*; Algora, A.*; Csatl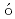 s, M.*; Deaven, J. M.*; Estevez-Aguado, E.*; Guess, C. J.*; et al.
s, M.*; Deaven, J. M.*; Estevez-Aguado, E.*; Guess, C. J.*; et al.
Physical Review C, 100(3), p.034618_1 - 034618_13, 2019/09
Times Cited Count:12 Percentile:77.09(Physics, Nuclear)no abstracts in English
Kakinouchi, Keisuke*; Nakamura, Tsutomu*; Tamada, Taro; Adachi, Hiroaki*; Sugiyama, Shigeru*; Maruyama, Mihoko*; Takahashi, Yoshinori*; Takano, Kazufumi*; Murakami, Satoshi*; Inoue, Tsuyoshi*; et al.
Journal of Applied Crystallography, 43(4), p.937 - 939, 2010/08
Times Cited Count:4 Percentile:48.31(Chemistry, Multidisciplinary)A method for growing large protein crystals is described. In this method, a cut pipette tip is used to hang large-scale droplets (maximum volume 200  l) consisting of protein and precipitating agents. A crystal grows at the vapor-liquid interface; thereafter the grown crystal can be retrieved by droplet-droplet contact both for repeated macroseeding and for mounting crystals in a capillary. Crystallization experiments with peroxiredoxin of
l) consisting of protein and precipitating agents. A crystal grows at the vapor-liquid interface; thereafter the grown crystal can be retrieved by droplet-droplet contact both for repeated macroseeding and for mounting crystals in a capillary. Crystallization experiments with peroxiredoxin of 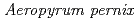 K1(thioredoxin peroxidase, ApTPx) and hen egg white lysozyme demonstrated that this large-scale hanging-drop method could produce a large-volume crystal very effectively. A neutron diffraction experiment confirmed that an ApTPx crystal (6.2 mm
K1(thioredoxin peroxidase, ApTPx) and hen egg white lysozyme demonstrated that this large-scale hanging-drop method could produce a large-volume crystal very effectively. A neutron diffraction experiment confirmed that an ApTPx crystal (6.2 mm ) obtained by this method diffracted to beyond 3.5
) obtained by this method diffracted to beyond 3.5  resolution.
resolution.
 resolution
resolutionShimizu, Noriko*; Sugiyama, Shigeru*; Maruyama, Mihoko*; Takahashi, Yoshinori*; Adachi, Motoyasu; Tamada, Taro; Hidaka, Koshi*; Hayashi, Yoshio*; Kimura, Toru*; Kiso, Yoshiaki*; et al.
Crystal Growth & Design, 10(7), p.2990 - 2994, 2010/06
Times Cited Count:11 Percentile:72.02(Chemistry, Multidisciplinary)We report crystal growth of human immunodeficiency virus 1 protease (HIV PR) in a complex with its inhibitor KNI-272 by six different methods. Comparative analysis indicates that top-seeded solution growth (TSSG) and TSSG combined with the floating and stirring technique (TSSG-FAST) are efficient strategies for rapidly obtaining large single crystals and effectively preventing polycrystallization of the seed crystal. Neutron diffraction analysis confirmed that the crystalobtained by TSSG is a high-quality single crystal. Furthermore, crystal shape was observed to be influenced by solution flow, suggesting that the degree of supersaturation significantly affects the crystal growth direction of HIV PR complex. This finding implies that the shape of the HIV PR complex crystal might be controlled by the solution flow rate.
Sakanaka, Shogo*; Akemoto, Mitsuo*; Aoto, Tomohiro*; Arakawa, Dai*; Asaoka, Seiji*; Enomoto, Atsushi*; Fukuda, Shigeki*; Furukawa, Kazuro*; Furuya, Takaaki*; Haga, Kaiichi*; et al.
Proceedings of 1st International Particle Accelerator Conference (IPAC '10) (Internet), p.2338 - 2340, 2010/05
Future synchrotron light source using a 5-GeV energy recovery linac (ERL) is under proposal by our Japanese collaboration team, and we are conducting R&D efforts for that. We are developing high-brightness DC photocathode guns, two types of cryomodules for both injector and main superconducting (SC) linacs, and 1.3 GHz high CW-power RF sources. We are also constructing the Compact ERL (cERL) for demonstrating the recirculation of low-emittance, high-current beams using above-mentioned critical technologies.
Adachi, Motoyasu; Ohara, Takashi; Kurihara, Kazuo; Tamada, Taro; Honjo, Eijiro; Okazaki, Nobuo; Arai, Shigeki; Shoyama, Yoshinari; Kimura, Kaname*; Matsumura, Hiroyoshi*; et al.
Proceedings of the National Academy of Sciences of the United States of America, 106(12), p.4641 - 4646, 2009/03
Times Cited Count:111 Percentile:90.72(Multidisciplinary Sciences)To further understand the catalytic mechanism and inhibitor recognition of HIV-1 protease, we need to determine the locations of key hydrogen atoms in the catalytic aspartates Asp25 and Asp125. The structure of HIV-1 protease in complex with transition-state analog KNI-272 was determined by combined neutron crystallography at 1.9  resolution and X-ray crystallography at 1.4
resolution and X-ray crystallography at 1.4  resolution. The resulting structural data shows that the catalytic residue Asp25 is protonated and that Asp125 is deprotonated. The proton on Asp25 makes a hydrogen bond with the carbonyl group of the allophenylnorstatine group in KNI-272. The deprotonated Asp125 bonds to the hydroxyl proton of Apns. The results provide direct experimental evidence for proposed aspects of the catalytic mechanism of HIV-1 protease; and can therefore contribute substantially to the development of specific inhibitors for therapeutic application.
resolution. The resulting structural data shows that the catalytic residue Asp25 is protonated and that Asp125 is deprotonated. The proton on Asp25 makes a hydrogen bond with the carbonyl group of the allophenylnorstatine group in KNI-272. The deprotonated Asp125 bonds to the hydroxyl proton of Apns. The results provide direct experimental evidence for proposed aspects of the catalytic mechanism of HIV-1 protease; and can therefore contribute substantially to the development of specific inhibitors for therapeutic application.
Matsumura, Hiroyoshi*; Adachi, Motoyasu; Sugiyama, Shigeru*; Okada, Shino*; Yamakami, Megumi*; Tamada, Taro; Hidaka, Koshi*; Hayashi, Yoshio*; Kimura, Toru*; Kiso, Yoshiaki*; et al.
Acta Crystallographica Section F, 64(11), p.1003 - 1006, 2008/11
Times Cited Count:17 Percentile:77.92(Biochemical Research Methods)This paper reports the crystallization and preliminary neutron diffraction measurements of HIV-1 protease, a potential target for anti-HIV therapy, complexed with an inhibitor (KNI-272). The aim of this neutron diffraction study is to obtain structural information about the H atoms and to determine the protonation states of the residues within the active site. The crystal was grown to a size of 1.4 mm by repeated macroseeding and a slow-cooling method using a two-liquid system. Neutron diffraction data were collected at room temperature using a BIX-4 diffractometer at the JRR-3 research reactor of the Japan Atomic Energy Agency (JAEA). The data set was integrated and scaled to 2.3
by repeated macroseeding and a slow-cooling method using a two-liquid system. Neutron diffraction data were collected at room temperature using a BIX-4 diffractometer at the JRR-3 research reactor of the Japan Atomic Energy Agency (JAEA). The data set was integrated and scaled to 2.3  resolution in space group P2(1)2(1)2, with unit-cell parameters a = 59.5, b = 87.4, c = 46.8
resolution in space group P2(1)2(1)2, with unit-cell parameters a = 59.5, b = 87.4, c = 46.8  .
.
Katayama, Masahito*; Adachi, Jun*; Kurosaki, Ken*; Osaka, Masahiko; Miwa, Shuhei; Tanaka, Kenya; Muta, Hiroaki*; Uno, Masayoshi*; Yamanaka, Shinsuke*
no journal, ,
no abstracts in English
Adachi, Motoyasu; Ohara, Takashi; Kurihara, Kazuo; Tamada, Taro; Honjo, Eijiro; Okazaki, Nobuo; Arai, Shigeki; Shoyama, Yoshinari; Matsumura, Hiroyoshi*; Sugiyama, Shigeru*; et al.
no journal, ,
We have determined a crystal structure of HIV-1 protease by neutron crystallography. The development of HIV-1 protease inhibitors is regarded as a major success of structure-based drug design and contributes to establish highly active anti-retroviral therapy for AIDS. To further understand the catalytic mechanism of HIV-1 protease and interaction between HIV-1 protease and its inhibitor, we have determined the crystal structure of HIV-1 protease in complex with a inhibitor, KNI-272 to 2.3  resolution by neutron crystallography. Our results indicates that the carbonyl group of allophenylnorstatine (Apns) in KNI-272 forms a significant hydrogen bond with protonated Asp 25, and the hydrogen atom from the hydroxyl group of Apns forms a remarkable hydrogen bond with the deprotonated Asp125. These results show direct evidence that Asp25 provides a proton to carbonyl group of substrate and Asp125 contributes to activate the attacking water molecule as a nucleophile.
resolution by neutron crystallography. Our results indicates that the carbonyl group of allophenylnorstatine (Apns) in KNI-272 forms a significant hydrogen bond with protonated Asp 25, and the hydrogen atom from the hydroxyl group of Apns forms a remarkable hydrogen bond with the deprotonated Asp125. These results show direct evidence that Asp25 provides a proton to carbonyl group of substrate and Asp125 contributes to activate the attacking water molecule as a nucleophile.
Adachi, Motoyasu; Ohara, Takashi; Kurihara, Kazuo; Tamada, Taro; Honjo, Eijiro; Okazaki, Nobuo; Arai, Shigeki; Shoyama, Yoshinari; Kimura, Kaname*; Matsumura, Hiroyoshi*; et al.
no journal, ,
HIV-1 protease is a dimeric aspartic protease that cleaves the nascent polyproteins of HIV-1 and plays an essential role in viral replication. To further understand the catalytic mechanism of HIV-1 protease, we have determined the crystal structure of HIV-1 protease in complex with a transition state mimetic tripeptide inhibitor, KNI-272 to 1.9  resolution by neutron crystallography in combination with 1.4
resolution by neutron crystallography in combination with 1.4  resolution X-ray diffraction data. Our results indicates that the carbonyl group of allophenylnorstatine in KNI-272 forms a significant hydrogen bond with protonated Asp 25, and the hydrogen atom from the hydroxyl group of Apns forms a remarkable hydrogen bond with the deprotonated Asp125. These results show direct evidence that Asp25 provides a proton to carbonyl group of substrate and Asp125 contributes to activate the attacking water molecule as a nucleophile.
resolution X-ray diffraction data. Our results indicates that the carbonyl group of allophenylnorstatine in KNI-272 forms a significant hydrogen bond with protonated Asp 25, and the hydrogen atom from the hydroxyl group of Apns forms a remarkable hydrogen bond with the deprotonated Asp125. These results show direct evidence that Asp25 provides a proton to carbonyl group of substrate and Asp125 contributes to activate the attacking water molecule as a nucleophile.
Adachi, Motoyasu; Ohara, Takashi; Kurihara, Kazuo; Tamada, Taro; Honjo, Eijiro; Okazaki, Nobuo; Arai, Shigeki; Shoyama, Yoshinari; Matsumura, Hiroyoshi*; Sugiyama, Shigeru*; et al.
no journal, ,
no abstracts in English
Adachi, Motoyasu; Ohara, Takashi; Kurihara, Kazuo; Tamada, Taro; Honjo, Eijiro*; Okazaki, Nobuo; Arai, Shigeki; Shoyama, Yoshinari*; Matsumura, Hiroyoshi*; Adachi, Hiroaki*; et al.
no journal, ,
To understand the catalytic mechanism of HIV-1 protease, we have determined the crystal structure of HIV-1 protease in complex with a transition state mimetic inhibitor, KNI-272 by neutron crystallography. Our results indicates that the carbonyl group of allophenylnorstatine in KNI-272 forms a significant hydrogen bond with protonated Asp 25, and the hydrogen atom from the hydroxyl group of allophenylnorstatine forms a remarkable hydrogen bond with the deprotonated Asp125. These results show direct evidence that Asp25 provides a proton to carbonyl group of substrate and Asp125 contributes to activate the attacking water molecule as a nucleophile.
Adachi, Motoyasu; Ohara, Takashi; Kurihara, Kazuo; Tamada, Taro; Honjo, Eijiro*; Okazaki, Nobuo; Arai, Shigeki; Shoyama, Yoshinari*; Matsumura, Hiroyoshi*; Sugiyama, Shigeru*; et al.
no journal, ,
In this study, we determined crystal structures of HIV-1 protease complexed with inhibitor by neutron and X-ray crystallography. Finally, we refined the structures to R-factor of 17.3% and free R-factor 20.3% by neutron crystallography and to R-factor of 10.4 % and free R-factor 12.4% by X-ray crystallography. The result shows that Asp 25 residue is protonated and Asp 125 is deprotonated. These information is important to resolve catalytic mechanism and design of new potent inhibitor.
Adachi, Motoyasu; Ohara, Takashi; Kurihara, Kazuo; Tamada, Taro; Honjo, Eijiro*; Okazaki, Nobuo; Arai, Shigeki; Shoyama, Yoshinari*; Matsumura, Hiroyoshi*; Adachi, Hiroaki*; et al.
no journal, ,
HIV-1 protease is a dimeric aspartic protease that cleaves the nascent polyproteins of HIV-1 and plays an essential role in viral replication. To further understand the catalytic mechanism of HIV-1 protease, we have determined the crystal structure of HIV-1 protease in complex with a transition state mimetic tripeptide inhibitor, KNI-272 to 1.9  ; resolution by neutron crystallography in combination with 1.4
; resolution by neutron crystallography in combination with 1.4  ; resolution X-ray diffraction data. Our results indicates that the carbonyl group of allophenylnorstatine (Apns) in KNI-272 forms a significant hydrogen bond with protonated Asp 25, and the hydrogen atom from the hydroxyl group of Apns forms a remarkable hydrogen bond with the deprotonated Asp125. These results show direct evidence that Asp25 provides a proton to carbonyl group of substrate and Asp125 contributes to activate the attacking water molecule as a nucleophile.
; resolution X-ray diffraction data. Our results indicates that the carbonyl group of allophenylnorstatine (Apns) in KNI-272 forms a significant hydrogen bond with protonated Asp 25, and the hydrogen atom from the hydroxyl group of Apns forms a remarkable hydrogen bond with the deprotonated Asp125. These results show direct evidence that Asp25 provides a proton to carbonyl group of substrate and Asp125 contributes to activate the attacking water molecule as a nucleophile.
Oba, Yojiro; Adachi, Nozomu*; Todaka, Yoshikazu*; Mamiya, Hiroaki*
no journal, ,
no abstracts in English
Yamazawa, Hiromi*; Sato, Yosuke*; Adachi, Shinichiro*; Takigawa, Masayuki*; Sekiyama, Tsuyoshi*; Kajino, Mizuo*; Terada, Hiroaki; Kondo, Hiroaki*; Uchida, Junya*; Goto, Daisuke*; et al.
no journal, ,
Cs-137 released from the Fukushima Daiichi Nuclear Power Plant (FDNPP) accident was conducted by 12 models. The present study focuses on differences in the model results of atmospheric Cs-137 concentration of Plume 2, which traveled southward in the morning of 15 March, 2011, in the area 100 to 200 km downwind from FDNPP by using the concentration data recently evaluated from gamma radiation spectral data at monitoring stations (MS data) and those measured from the suspended particulate matter filters (SPM data). Comparison was made from the following aspects: (1) plume arrival time, (2) concentration level, (3) cross-wind surface concentration profile, (4) vertical concentration profile and (5) mass balance of Cs-137 activity including deposition processes. Additional analyses were made also for Plume 4, which traveled over the same area on 16 March under rainy condition.
Yamazawa, Hiromi*; Sato, Yosuke*; Oura, Yasuji*; Moriguchi, Yuichi*; Terada, Hiroaki; Furuno, Akiko; Tsuzuki, Katsunori; Kadowaki, Masanao; Sekiyama, Tsuyoshi*; Adachi, Koji*; et al.
no journal, ,
no abstracts in English