Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ishii, Yasuo; Toyoshima, Atsushi; Tsukada, Kazuaki; Asai, Masato; Li, Z.*; Nagame, Yuichiro; Miyashita, Sunao*; Mori, Tomotaka*; Suganuma, Hideo*; Haba, Hiromitsu*; et al.
Bulletin of the Chemical Society of Japan, 84(9), p.903 - 911, 2011/09
Times Cited Count:17 Percentile:49.33(Chemistry, Multidisciplinary)The cation-exchange behavior of element 104, rutherfordium (Rf), was investigated together with its lighter group-4 homologues Zr and Hf, and the tetravalent pseudo-homologue Th in HF/HNO mixed solution. The results demonstrate that distribution coefficients (
mixed solution. The results demonstrate that distribution coefficients (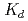 ) of Rf in HF/0.10 M HNO
) of Rf in HF/0.10 M HNO decrease with increasing concentration of the fluoride ion [F
decrease with increasing concentration of the fluoride ion [F ], indicating the consecutive formation of fluorido complexes of Rf. We also measured the
], indicating the consecutive formation of fluorido complexes of Rf. We also measured the 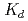 values of Rf and the homologues as a function of the hydrogen ion concentration [H
values of Rf and the homologues as a function of the hydrogen ion concentration [H ]. The log
]. The log 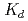 values decrease linearly with an increase of log [H
values decrease linearly with an increase of log [H ] with slopes between -2.1 and -2.5. This indicates that these elements are likely to form the same chemical compounds: mixture of [MF]
] with slopes between -2.1 and -2.5. This indicates that these elements are likely to form the same chemical compounds: mixture of [MF] and [MF
and [MF ]
] (M = Rf, Zr, Hf and Th) in the studied solution. It is also ascertained that sequence in the fluoride complex formation is Zr
(M = Rf, Zr, Hf and Th) in the studied solution. It is also ascertained that sequence in the fluoride complex formation is Zr  Hf
Hf  Rf
Rf  Th.
Th.
Ishii, Yasuo; Toyoshima, Atsushi; Tsukada, Kazuaki; Asai, Masato; Tome, Hayato; Nishinaka, Ichiro; Nagame, Yuichiro; Miyashita, Sunao*; Mori, Tomotaka*; Suganuma, Hideo*; et al.
Chemistry Letters, 37(3), p.288 - 289, 2008/03
Times Cited Count:20 Percentile:55.03(Chemistry, Multidisciplinary)We have investigated cation-exchange behavior of Rf together with the lighter homologues of the group-4 elements Zr and Hf, and the tetravalent pseudo-homologue Th, in HF/HNO solution using Automated Ion exchange separation apparatus coupled with the Detection system for Alpha spectroscopy (AIDA). The
solution using Automated Ion exchange separation apparatus coupled with the Detection system for Alpha spectroscopy (AIDA). The 
 values of Zr, Hf, Th and Rf in HF/0.1 M HNO
values of Zr, Hf, Th and Rf in HF/0.1 M HNO were decreased with increasing the concentration of the fluoride ion [F
were decreased with increasing the concentration of the fluoride ion [F ], indicating the formation of the fluoride complexes. The sequence of the fluoride complexation strength is Zr
], indicating the formation of the fluoride complexes. The sequence of the fluoride complexation strength is Zr  Hf
Hf  Rf
Rf  Th.
Th.
Okuno, Hiroshi; Akiyama, Hideo*; Mochizuki, Hiroki*
Journal of Nuclear Science and Technology, 40(1), p.57 - 60, 2003/01
Times Cited Count:1 Percentile:10.88(Nuclear Science & Technology)Low-level waste (LLW) drums are required to transport as fissile material if the current IAEA's Regulations for the Safe Transport of Radioactive Material are rigorously applied. This problem is a consequence that water contents of concrete in LLW drums contained deuterium (D) in quantities more than 0.1% of fissile material mass, therefore they are not excepted from packages containing fissile material. Consideration of differences in the absorption cross sections of light hydrogen and D shows that the relative increase in the neutron multiplication factor by a presence of D in natural water for hydrogen (H)-moderated systems is not larger than 0.015%. A numerical calculation confirms that the infinite multiplication factor of a mixture of  U-metal and water in a
U-metal and water in a  U/H mass ratio of 5% increases proportionally to the D/H atomic ratio, and that its relative increase is less than 0.03% for the D/H atomic ratio of 0.015%. The limiting fissile-to-H mass ratio of 5% in the exception rule is concluded to be applicable to H-moderated systems including D in natural water.
U/H mass ratio of 5% increases proportionally to the D/H atomic ratio, and that its relative increase is less than 0.03% for the D/H atomic ratio of 0.015%. The limiting fissile-to-H mass ratio of 5% in the exception rule is concluded to be applicable to H-moderated systems including D in natural water.
Matsuda, Hideo*; Omura, Ichiro*; Sakiyama, Yoko*; Urano, Satoshi*; Iesaka, Susumu*; Ohashi, Hiromichi*; Hirao, Toshio; Abe, Hiroshi; Ito, Hisayoshi; Mori, Hidenobu; et al.
JAERI-Review 2002-035, TIARA Annual Report 2001, p.11 - 13, 2002/11
no abstracts in English
Kumamaru, Hiroshige; ; Murata, Hideo; Kukita, Yutaka; Akiyama, Mamoru*; *; *; *; *; *; et al.
Proc. of ASME JSME 4th Int. Conf. on Nuclear Engineering 1996 (ICONE-4), 1(PART B), p.669 - 674, 1996/00
JSME 4th Int. Conf. on Nuclear Engineering 1996 (ICONE-4), 1(PART B), p.669 - 674, 1996/00
no abstracts in English
 /HF mixed solution, 2
/HF mixed solution, 2Ishii, Yasuo; Tome, Hayato; Toyoshima, Atsushi; Asai, Masato; Nishinaka, Ichiro; Tsukada, Kazuaki; Nagame, Yuichiro; Miyashita, Sunao*; Mori, Tomotaka*; Suganuma, Hideo*; et al.
no journal, ,
Chemical properties of rutherfordium (Rf, the element 104) in HNO /HF mixed solutions have been investigated using cation-exchange chromatography. Distribution coefficients of Rf on the cation-exchange resin as a function of hydrofluoric-acid and nitric-acid concentrations have been measured precisely, and it was found that the strength of the fluoride complex formation of Rf is much weaker than those of lighter group 4 homologs Zr and Hf. The same result was obtained through the anion-exchange chromatography. These results enabled us to quantitatively account for the fluoride complexation of Rf.
/HF mixed solutions have been investigated using cation-exchange chromatography. Distribution coefficients of Rf on the cation-exchange resin as a function of hydrofluoric-acid and nitric-acid concentrations have been measured precisely, and it was found that the strength of the fluoride complex formation of Rf is much weaker than those of lighter group 4 homologs Zr and Hf. The same result was obtained through the anion-exchange chromatography. These results enabled us to quantitatively account for the fluoride complexation of Rf.
 /HF solution
/HF solutionIshii, Yasuo; Tome, Hayato; Toyoshima, Atsushi; Asai, Masato; Nishinaka, Ichiro; Tsukada, Kazuaki; Nagame, Yuichiro; Miyashita, Sunao*; Mori, Tomotaka*; Suganuma, Hideo*; et al.
no journal, ,
The cation-exchange behavior of rutherfordium(Rf) in HNO /HF solution has been studied based on an atom-at-a-time scale. In the symposium, we will discuss the fluoride complexation of rutherfordium in comparison with that of Zr, Hf and Th.
/HF solution has been studied based on an atom-at-a-time scale. In the symposium, we will discuss the fluoride complexation of rutherfordium in comparison with that of Zr, Hf and Th.
Unno, Motoyoshi; Ebashi, Tsutomu; Kawasaki, Takashi; Tomotsune, Yusuke; Akiyama, Kiyomitsu; Ejiri, Hideo; Kobayashi, Hirohide
no journal, ,
no abstracts in English