Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ikeda, Takashi; Hou, Z.*; Chai, G.-L.*; Terakura, Kiyoyuki*
Hyomen Kagaku, 36(7), p.345 - 350, 2015/07
Carbon alloy catalysts (CACs) are one of promising candidates for platinum-substitute cathode catalysts for polymer electrolyte fuel cells. We have investigated possible mechanisms of oxygen reduction reactions (ORRs) for CACs via first-principles-based molecular dynamics simulations. In this contribution, we review possible ORRs at likely catalytic sites of CACs suggested from our simulations.
Chai, G.-L.*; Hou, Z.*; Shu, D.-J.*; Ikeda, Takashi; Terakura, Kiyoyuki*
Journal of the American Chemical Society, 136(39), p.13629 - 13640, 2014/10
Times Cited Count:253 Percentile:97.75(Chemistry, Multidisciplinary)Carbon alloy catalysts (CACs) are promising catalysts for oxygen reduction reaction (ORR) to substitute Pt. However, despite extensive studies on CACs the reaction sites and mechanisms for ORR are still in controversy. Herein, we present rather general consideration on possible ORR mechanisms for various structures in nitrogen doped CACs based on the first principles calculations. Our study indicates that only a particular structure of a nitrogen pair doped Stone-Wales defect provides a good active site. The ORR activity of this structure can be tuned by the curvature around the active site, which makes its limiting potential approaching the maximum limiting potential (0.80 V) in the volcano plot for the ORR activity of CACs. The calculated results can be compared with the recent experimental ones of the half wave potential for CAC systems that range from 0.60 V to 0.80 V in the reversible-hydrogen-electrode scale.
Ikeda, Takashi; Hou, Z.*; Chai, G.-L.*; Terakura, Kiyoyuki*
Journal of Physical Chemistry C, 118(31), p.17616 - 17625, 2014/08
Times Cited Count:51 Percentile:80.6(Chemistry, Physical)N-doped carbon-based nanomaterials are attracting a great interest as promising Pt-free electrode catalysts for polymer electrolyte fuel cells (PEFCs). In this computational study, we demonstrate that N-doped graphene edges can exhibit enhanced catalytic activity toward oxygen reduction reactions by controlling their electron-donating and -withdrawing abilities, and basicity, resulting in higher selectivity of 4e reduction via inner and outer sphere electron transfer at edges in acidic conditions, respectively. Our simulations also show that 2e
reduction via inner and outer sphere electron transfer at edges in acidic conditions, respectively. Our simulations also show that 2e reduction occurs selectively in the presence of pyridinic N next to carbonyl O at zigzag edges. This study thus rationalizes the roles of doped N in graphenelike materials for oxygen reduction reactions.
reduction occurs selectively in the presence of pyridinic N next to carbonyl O at zigzag edges. This study thus rationalizes the roles of doped N in graphenelike materials for oxygen reduction reactions.
Hou, Z.*; Shu, D.-J.*; Chai, G.-L.*; Ikeda, Takashi; Terakura, Kiyoyuki*
Journal of Physical Chemistry C, 118(34), p.19795 - 19805, 2014/08
Times Cited Count:11 Percentile:35.66(Chemistry, Physical)In most of the N-doped graphene which attracts strong attention in the context of precious-metal free catalysts and nanoelectronics, the oxygen content is generally higher than or at least comparable to the nitrogen content. We perform density functional theory calculations to study the interplay of oxidized monovacancies and the nitrogen doping, motivated by the fact that MV is more frequently observed and more chemically active than divacancy and Stone-Wales defect. We determine the phase diagrams of un-doped and nitrogen-doped oxidized MVs as a function of temperature and partial pressure of O and H
and H gases. The modification of the electronic structure of MV by oxidation and N doping is studied. Our results show that the ether group is a common component in stable configurations of oxidized MVs. Most of the stable configurations of oxidized MVs do not induce any carriers.
gases. The modification of the electronic structure of MV by oxidation and N doping is studied. Our results show that the ether group is a common component in stable configurations of oxidized MVs. Most of the stable configurations of oxidized MVs do not induce any carriers.
Shi, W.-Q.*; Fu, H.-Y.*; Bounds, P. L.*; Muroya, Yusa*; Lin, M.; Katsumura, Yosuke*; Zhao, Y.-L.*; Chai, Z.-F.*
Radiation Research, 176(1), p.128 - 133, 2011/07
Times Cited Count:3 Percentile:17.14(Biology)3-Nitrotyrosine (3-NT) has been reported as an important biomarker of oxidative stress and potential source of reactive oxygen species (ROSs). In this work, the UV-visible absorption spectra of the transients formed by hydrated electron (e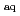
 ) reacting with 3-NT and its derivatives were investigated, the spectra showed many characteristics of aromatic nitro anion radical. The reaction rate constants of e
) reacting with 3-NT and its derivatives were investigated, the spectra showed many characteristics of aromatic nitro anion radical. The reaction rate constants of e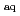
 reacting with 3-NT, N-acetyl-3-nitrotyrosine ethyl ester (NANTE) and nitrotyrosine-containing peptide Gly-nitroTyr-Gly at neutral pH were determined, respectively, which were almost two orders of magnitude higher than that of tyrosine and tyrosine-containing peptides. The pH-dependence of e
reacting with 3-NT, N-acetyl-3-nitrotyrosine ethyl ester (NANTE) and nitrotyrosine-containing peptide Gly-nitroTyr-Gly at neutral pH were determined, respectively, which were almost two orders of magnitude higher than that of tyrosine and tyrosine-containing peptides. The pH-dependence of e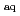
 decay rate constants in presence of 3-NT was also studied.
decay rate constants in presence of 3-NT was also studied.
Lin, M.; Shi, W.*; Fu, H.*; Muroya, Yusa*; Katsumura, Yosuke; Xu, D.*; Chai, Z.*
no journal, ,
The reaction between reactive nitrogen species (RNSs) such as peroxynitrite and nitrogen oxide (NOx), and aromatic amino acids such as tyrosine and tryptophan results in a number of modifications, of which tyrosine nitration has gained much attention. 3-Nitrotyrosine (3-NT) was considered as an important biomarker of nitrative stress  . In this work, the reaction rate constants of 3-NT, N-acetyl-3-nitrotyrosine ethyl ester (NANTE) and 3-NT containing peptide Gly-nitroTyr-Gly reacting with azide radical (N
. In this work, the reaction rate constants of 3-NT, N-acetyl-3-nitrotyrosine ethyl ester (NANTE) and 3-NT containing peptide Gly-nitroTyr-Gly reacting with azide radical (N
 ) at pH 6.0 were measured. The UV-visible absorption spectra of the transients of N
) at pH 6.0 were measured. The UV-visible absorption spectra of the transients of N
 reacting with the above compounds were also investigated. The results implied the possibility of protein aggregation through covalent dimerization of 3-NT residue
reacting with the above compounds were also investigated. The results implied the possibility of protein aggregation through covalent dimerization of 3-NT residue  .
.
Ikeda, Takashi; Chai, G.*; Hou, Z.*; Shu, D.*; Terakura, Kiyoyuki*
no journal, ,
no abstracts in English
Ikeda, Takashi; Chai, G.*; Hou, Z.*; Terakura, Kiyoyuki*
no journal, ,
Polymer electrolyte fuel cells are one of the most promising power sources. However, their practical use continues to be hindered by the prohibitive cost of Pt-based catalysts required to facilitate electrode reactions at operating temperatures of 80 C. Recently, a large number of groups have reported significantly high ORR activities of sp
C. Recently, a large number of groups have reported significantly high ORR activities of sp carbon-based materials doped with light elements such as N, B, S, etc., thus leading to much debate on the role of the doped light elements in the ORR activity. In this computational study, we inspect possible oxygen adsorption and reduction processes on various models of N-doped defective graphene using FPMD simulations. The dynamics of an O
carbon-based materials doped with light elements such as N, B, S, etc., thus leading to much debate on the role of the doped light elements in the ORR activity. In this computational study, we inspect possible oxygen adsorption and reduction processes on various models of N-doped defective graphene using FPMD simulations. The dynamics of an O molecule solvated in water along with energetic considerations, indicates that the N doping in defective graphenes can enhance efficiently their catalytic activity depending on the detailed structure of defects as well as the position of N dopants.
molecule solvated in water along with energetic considerations, indicates that the N doping in defective graphenes can enhance efficiently their catalytic activity depending on the detailed structure of defects as well as the position of N dopants.
Ikeda, Takashi; Chai, G.*; Hou, Z.*; Terakura, Kiyoyuki*
no journal, ,
no abstracts in English
Ikeda, Takashi; Chai, G.*; Hou, Z.*; Terakura, Kiyoyuki*
no journal, ,
Recently, carbon-based nanomaterials doped with heteroatoms such as N are attracting a great interest as promising Pt-free electrode catalysts for polymer electrolyte fuel cells. For further enhancing their catalytic activity it is of crucial importance to identify catalytic sites of carbon-based materials and to elucidate reaction mechanisms at atomistic level. In this computational study, we inspect possible oxygen adsorption and reduction processes on various models of N-doped graphene using first principles-based molecular dynamics simulations. In this talk we summarize possible paths of oxygen reduction reaction and catalytic activity for N-doped carbon alloy catalysts suggested from our simulations.
Ikeda, Takashi; Hou, Z.*; Chai, G.-L.*; Terakura, Kiyoyuki*
no journal, ,
no abstracts in English