Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 C
CMaeda, Toshikatsu; Chiba, Noriaki; Tateishi, Tsuyoshi*; Yamaguchi, Tetsuji
Nihon Genshiryoku Gakkai Wabun Rombunshi, 12(2), p.158 - 164, 2013/06
no abstracts in English
Yamashita, Hiromasa*; Oka, Kiyoshi; Yamanaka, Noriaki*; Seki, Takeshi; Kim, K.*; Kuwana, Kenta*; Masamune, Ken*; Naganawa, Akihiro*; Dohi, Takeyoshi*; Chiba, Toshio*
Nihon Reza Igakkai-Shi, 33(2), p.122 - 130, 2012/08
no abstracts in English
Moriyama, Kiyofumi; Tashiro, Shinsuke; Chiba, Noriaki; Maruyama, Yu; Nakamura, Hideo; Watanabe, Atsushi*
JAEA-Research 2011-016, 125 Pages, 2011/06
The volatile iodine production due to radiation chemical effects in the containment vessel of light water reactors (LWRs) during severe accidents was investigated by experiments in small scale and with well controlled conditions. Cesium iodide solutions, 10 M, labeled with
M, labeled with  I, at controlled pH by boric acid-sodium hydroxide buffer, were
I, at controlled pH by boric acid-sodium hydroxide buffer, were  -irradiated and swept with a constant gas flow rate. The gaseous iodine released from the solution was collected by species selective filters and quantified separately for I
-irradiated and swept with a constant gas flow rate. The gaseous iodine released from the solution was collected by species selective filters and quantified separately for I and organic iodines. The influences of pH, temperature, inorganic and organic impurities, oxygen and hydrogen concentrations in the cover gas on the iodine release behavior were examined. Data including time dependent gaseous iodine release fractions, comparison of the final iodine release fractions in terms of the parameter effects, as well as the initial, boundary and interface conditions necessary for simulating the experiments by computer codes are provided.
and organic iodines. The influences of pH, temperature, inorganic and organic impurities, oxygen and hydrogen concentrations in the cover gas on the iodine release behavior were examined. Data including time dependent gaseous iodine release fractions, comparison of the final iodine release fractions in terms of the parameter effects, as well as the initial, boundary and interface conditions necessary for simulating the experiments by computer codes are provided.
Moriyama, Kiyofumi; Chiba, Noriaki; Tashiro, Shinsuke; Maruyama, Yu; Nakamura, Hideo; Watanabe, Atsushi*
Journal of Nuclear Science and Technology, 48(6), p.885 - 891, 2011/06
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)The leach of remaining solvent in an epoxy paint coating when it is submerged in water was experimentally studied. A leach kinetics model considering the equilibrium of the solvent concentration in the paint matrix and in water was developed. Three model parameters, equilibrium constant  , leaching rate
, leaching rate 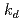 , and initial concentration of the solvent in the paint
, and initial concentration of the solvent in the paint 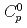 were evaluated based on the experimental results, and empirical correlation equations for them were obtained. The model showed good qualitative and quantitative agreement with the observed evolution of the leached solvent mass in the present experiment. Also the model showed consistency with experimental data by Ball et al. (2003).
were evaluated based on the experimental results, and empirical correlation equations for them were obtained. The model showed good qualitative and quantitative agreement with the observed evolution of the leached solvent mass in the present experiment. Also the model showed consistency with experimental data by Ball et al. (2003).
 -irradiated aqueous CsI solution and influence of oxygen and Methyl Isobutyl Ketone (MIBK)
-irradiated aqueous CsI solution and influence of oxygen and Methyl Isobutyl Ketone (MIBK)Moriyama, Kiyofumi; Tashiro, Shinsuke; Chiba, Noriaki; Hirayama, Fumio*; Maruyama, Yu; Nakamura, Hideo; Watanabe, Atsushi*
Journal of Nuclear Science and Technology, 47(3), p.229 - 237, 2010/03
Times Cited Count:19 Percentile:75.78(Nuclear Science & Technology)The volatile iodine production due to radiation chemistry is an important uncertainty source in the source term evaluation for LWRs. The gaseous release of molecular iodine and organic iodine from  -irradiated (
-irradiated (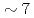 kGy/h, 2h) cesium iodide aqueous solution (1E-4M) containing methyl-isobuthyl-ketone (MIBK) was measured. The solution was buffered at pH
kGy/h, 2h) cesium iodide aqueous solution (1E-4M) containing methyl-isobuthyl-ketone (MIBK) was measured. The solution was buffered at pH 7. The concentration of MIBK (up to 1E-3M) and oxygen were changed as parameters. The total iodine release fraction and the fraction released as organic iodine were 2-47% and 0.02-1.5%, respectively, at the end of the irradiation. With the same cover gas condition, the total iodine release decreased and the organic iodine release increased when the MIBK concentration increased. This behavior can be explained by branching of the reaction path of organic degradation depending on availability of dissolved oxygen and competition between iodine and organic compounds on the consumption of radicals.
7. The concentration of MIBK (up to 1E-3M) and oxygen were changed as parameters. The total iodine release fraction and the fraction released as organic iodine were 2-47% and 0.02-1.5%, respectively, at the end of the irradiation. With the same cover gas condition, the total iodine release decreased and the organic iodine release increased when the MIBK concentration increased. This behavior can be explained by branching of the reaction path of organic degradation depending on availability of dissolved oxygen and competition between iodine and organic compounds on the consumption of radicals.
Moriyama, Kiyofumi; Tashiro, Shinsuke; Chiba, Noriaki; Nakamura, Hideo; Nakamura, Koichi*
no journal, ,
Experiments were performed to measure the gaseous iodine release from aqueous CsI solution irradiated by Co-60  -ray, to clarify the gaseous iodine behavior in the containment of light water reactors during severe accidents (SA). The influence of organic compounds with various oxygen concentrations in the gas phase were examined with pH
-ray, to clarify the gaseous iodine behavior in the containment of light water reactors during severe accidents (SA). The influence of organic compounds with various oxygen concentrations in the gas phase were examined with pH 7 at room temperature.
7 at room temperature.
Moriyama, Kiyofumi; Chiba, Noriaki; Tashiro, Shinsuke; Nakamura, Hideo; Nakamura, Koichi*
no journal, ,
Regarding the organic iodine production during a severe accident of light water reactors, the organic solvent leaching from paints has drawn attention as an important organic source. We measured the amount of organic solvent leaching from epoxy paint as time series data and examined its dependence on temperature, paint thickness and age. We developed also a leach behavior model considering equilibrium between the concentrations in the paint matrix and in the solution.
Moriyama, Kiyofumi; Chiba, Noriaki; Tashiro, Shinsuke; Nakamura, Hideo; Nakamura, Koichi*
no journal, ,
no abstracts in English
 C under anaerobic condition
C under anaerobic conditionChiba, Noriaki; Maeda, Toshikatsu; Yamaguchi, Tetsuji
no journal, ,
no abstracts in English
Chiba, Noriaki; Maeda, Toshikatsu; Yamaguchi, Tetsuji
no journal, ,
no abstracts in English
 C
CChiba, Noriaki; Maeda, Toshikatsu; Yamaguchi, Tetsuji
no journal, ,
no abstracts in English
 C; Study on corrosion mechanisms using isotope effects in hydrogen generation process
C; Study on corrosion mechanisms using isotope effects in hydrogen generation processChiba, Noriaki; Yamaguchi, Tetsuji; Maeda, Toshikatsu
no journal, ,
no abstracts in English
Shimoyama, Iwao; Honda, Mitsunori; Kogure, Toshihiro*; Baba, Yuji; Okamoto, Yoshihiro; Chiba, Noriaki; Yaita, Tsuyoshi; Suzuki, Shinichi
no journal, ,
We have found that Cs can be largely removed from weathered biotite (WB) in Fukushima by low-pressure heating treatment with some alkaline salts, but the detail of the mechanism is still unclear. We analyze compositional and structural change of WB using XRF, XRD, TEM, and NEXAFS. NaCl-CaCl mixed salt and WB were mixed in a weight ratio of 1/1 and heated in a low-pressure condition. Excess salt in the sample was removed by rinsing with distilled water. Cs/Si ratio decreased to 11% and 0 % by the treatment at 600
mixed salt and WB were mixed in a weight ratio of 1/1 and heated in a low-pressure condition. Excess salt in the sample was removed by rinsing with distilled water. Cs/Si ratio decreased to 11% and 0 % by the treatment at 600 C and 700
C and 700 C, respectively. We found decrease of K with Cs. Meanwhile, Ca and Cl increased with temperature. XRD analysis clarified that new peaks appeared above 500
C, respectively. We found decrease of K with Cs. Meanwhile, Ca and Cl increased with temperature. XRD analysis clarified that new peaks appeared above 500 C and peaks of WB disappeared at 700
C and peaks of WB disappeared at 700 C. TEM analysis clarified that augite and wadalite were dominant products after the heating treatment. NEXAFS analysis clarified that Ca and Cl have different chemical bonding depending on atmospheric and low pressures. Based on these results, we propose Cs free mineralization for decontamination of soil in Fukushima.
C. TEM analysis clarified that augite and wadalite were dominant products after the heating treatment. NEXAFS analysis clarified that Ca and Cl have different chemical bonding depending on atmospheric and low pressures. Based on these results, we propose Cs free mineralization for decontamination of soil in Fukushima.