Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nakayama, Shinichi; Okumura, Masahiko*; Nagasaki, Shinya*; Enokida, Yoichi*; Umeki, Hiroyuki*; Takase, Hiroyasu*; Kawasaki, Daisuke*; Hasegawa, Shuichi*; Furuta, Kazuo*
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 23(2), p.131 - 148, 2016/12
A symposium "Science of nuclear fuel cycle and backend - Research and education -" was held at the Univer-sity of Tokyo in June 25, 2016. This aimed at developing the research on nuclear fuel cycle and backend. The time and the number of participants of the symposium were limited, but the active discussion was conducted, and the common perception for the future was shared among the experienced participants in those fields. This paper provides the discussions made in the symposium, and also, as a memory to Professor Ahn, the University of California, Berkeley, his prominent achievements in academic research and education.
Suzuki, Yoshinori; Nankawa, Takuya; Ozaki, Takuo; Onuki, Toshihiko; Francis, A. J.*; Enokida, Yoichi*; Yamamoto, Ichiro*
Journal of Nuclear Science and Technology, 44(9), p.1227 - 1232, 2007/09
Times Cited Count:16 Percentile:72.04(Nuclear Science & Technology)We examined electrochemical redox reactions of UO
 in organic acid (oxalic, malonic, succinic, adipic, L-malic, and L-tartaric acids) solutions using cyclic voltammetry. A redox reaction of UO
in organic acid (oxalic, malonic, succinic, adipic, L-malic, and L-tartaric acids) solutions using cyclic voltammetry. A redox reaction of UO
 /UO
/UO
 and an oxidation reaction of U(IV) were observed. The peak potentials of the UO
and an oxidation reaction of U(IV) were observed. The peak potentials of the UO
 reduction showed a good linear relationship with the log of the stability constants of 1:1 UO
reduction showed a good linear relationship with the log of the stability constants of 1:1 UO
 -organic complexes. We also revealed the redox reactions of UO
-organic complexes. We also revealed the redox reactions of UO
 in the presence of malonic or oxalic acids between pH 1 and 6.
in the presence of malonic or oxalic acids between pH 1 and 6.
Suzuki, Yoshinori; Nankawa, Takuya; Yoshida, Takahiro*; Ozaki, Takuo; Onuki, Toshihiko; Francis, A. J.; Tsushima, Satoru*; Enokida, Yoichi*; Yamamoto, Ichiro*
Radiochimica Acta, 94(9-11), p.579 - 583, 2006/11
Times Cited Count:20 Percentile:78.89(Chemistry, Inorganic & Nuclear)We examined the reduction behavior of UO
 in citrate media at pH 2.0-7.0 by column electrodes. At pH 2.0, UO
in citrate media at pH 2.0-7.0 by column electrodes. At pH 2.0, UO
 was reduced to U(IV) through a one-step reduction process, while it was reduced to U(IV) through a two-step reduction process at pH 3.0-5.0. The reduction potential of UO
was reduced to U(IV) through a one-step reduction process, while it was reduced to U(IV) through a two-step reduction process at pH 3.0-5.0. The reduction potential of UO
 shifted to lower values with an increase in pH from 2.0 to 7.0. At pH 6.0 and 7.0, UO
shifted to lower values with an increase in pH from 2.0 to 7.0. At pH 6.0 and 7.0, UO
 was not reduced to U(IV) completely at the electrode potential above -0.8 V. Ultraviolet-visible spectroscopy and speciation calculation of UO
was not reduced to U(IV) completely at the electrode potential above -0.8 V. Ultraviolet-visible spectroscopy and speciation calculation of UO
 in citrate media indicated that uranium existed as a mainly UO
in citrate media indicated that uranium existed as a mainly UO
 at pH 2-3, and a predominant species at pH 3-5 was [(UO
at pH 2-3, and a predominant species at pH 3-5 was [(UO )
) Cit
Cit ]
] . At pH 5-7, polymeric complexes were present. These findings suggest that the reduction of UO
. At pH 5-7, polymeric complexes were present. These findings suggest that the reduction of UO
 is more difficult by polymerization of UO
is more difficult by polymerization of UO
 with citric acid at higher pHs.
with citric acid at higher pHs.
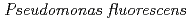 in the presence of citric acid
in the presence of citric acidSuzuki, Yoshinori; Nankawa, Takuya; Yoshida, Takahiro*; Ozaki, Takuo; Onuki, Toshihiko; Francis, A. J.*; Tsushima, Satoru*; Enokida, Yoichi*; Yamamoto, Ichiro*
Journal of Nuclear and Radiochemical Sciences, 6(1), p.91 - 93, 2005/07
We studied the sorption of Eu(III) on Pseudomonas fluorescens in the absence and presence of citric acid by a batch method. The cells were placed in a solution containing 0.002 mM of Eu(III) and 0, 0.1, or 1 mM of citric acid at pH 3-9 for 5 hours. In the absence of citric acid, almost 100 % of Eu(III) was sorbed on P. fluorescens at pHs below 7; above 7, sorption decreased with an increase in pH. The time course of Eu(III) sorption on P. fluorescens showed that a fraction of it was desorbed into the solution at alkaline pHs, suggesting that the bacterium may release some exudates. With citric acid present, we found that at higher concentrations there was lower sorption of Eu(III), reflecting the formation of Eu(III)-citrate complexes competes with the Eu(III)-cell-surface complexes. This decrease in Eu(III) sorption was significant in alkaline pHs.
Iguchi, Tetsuo*; Watanabe, Kenichi*; Kawarabayashi, Jun*; Uritani, Akira*; Enokida, Yoichi*; Watanabe, Kazuo
JAERI-Tech 2004-010, 62 Pages, 2004/03
no abstracts in English
 Complex for Super-DIREX Process
Complex for Super-DIREX ProcessKamiya, Masayoshi; Miura, Sachiko; Nomura, Kazunori; Koyama, Tomozo; Ogumo, Shinya*; Mori, Yukihide*; Enokida, Yoichi*
CD-ROM, P1-35, 4P., 4 Pages, 2004/00
Super-DIREX is a new reprocessing method which has high economical efficiency. Experimental study of this process was started on the direct extraction of U and Pu from irradiated MOX fuel by the supercritical carbon dioxide (SFCO ) containing TBP-HNO
) containing TBP-HNO complex. This report describes direct extraction of U and Pu with TBP-HNO3 complex at atmospheric pressure, as the first test for irradiated fuel, in order to investigate the applicability of SFCO
complex. This report describes direct extraction of U and Pu with TBP-HNO3 complex at atmospheric pressure, as the first test for irradiated fuel, in order to investigate the applicability of SFCO containing TBP-HNO
containing TBP-HNO complex. In this test, dependency on dissolution temperature, Pu content, fuel/ TBP-HNO
complex. In this test, dependency on dissolution temperature, Pu content, fuel/ TBP-HNO complex ratio and effect of voloxidation were investigated. From these results, TBP-HNO
complex ratio and effect of voloxidation were investigated. From these results, TBP-HNO complex was found to be effective in the respect of the recovery of U and Pu. The number of the process step in dissolution and co-extraction is small, and amount of waste can be reduced. It is applicable to the direct extraction in Super-DIREX.
complex was found to be effective in the respect of the recovery of U and Pu. The number of the process step in dissolution and co-extraction is small, and amount of waste can be reduced. It is applicable to the direct extraction in Super-DIREX.
 fluid leaching (SFL) of uranium from solid wastes using HNO
fluid leaching (SFL) of uranium from solid wastes using HNO -TBP complex as a reactant
-TBP complex as a reactantTomioka, Osamu*; Meguro, Yoshihiro; Iso, Shuichi; Yoshida, Zenko; Enokida, Yoichi*; Yamamoto, Ichiro*
Proceedings of International Solvent Extraction Conference 2002 (CD-ROM), p.1143 - 1147, 2002/00
no abstracts in English
 medium containing HNO
medium containing HNO -TBP complex
-TBP complexTomioka, Osamu*; Meguro, Yoshihiro; Iso, Shuichi; Yoshida, Zenko; Enokida, Yoichi*; Yamamoto, Ichiro*
Journal of Nuclear Science and Technology, 38(6), p.461 - 462, 2001/06
Times Cited Count:27 Percentile:85.72(Nuclear Science & Technology)no abstracts in English
 complex
complexMiyahara, Sachiko; Funasaka, Hideyuki; Enokida, Yoichi*; Yamamoto, Ichiro*
ATALANTE2000, 0 Pages, 2000/00
None
Suzuki, Yoshinori; Enokida, Yoichi*; Yamamoto, Ichiro*; Nankawa, Takuya; Ozaki, Takuo; Onuki, Toshihiko
no journal, ,
no abstracts in English