Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Be concentrations in bed-sediments of Ado and Yasu rivers flowing into Lake Biwa
Be concentrations in bed-sediments of Ado and Yasu rivers flowing into Lake BiwaFujisawa, Jumpei*; Minami, Masayo*; Kokubu, Yoko; Matsuzaki, Hiroyuki*
JAEA-Conf 2018-002, p.91 - 94, 2019/02
Beryllium-10 ( Be) of a cosmogenic nuclide precipitates in forms of BeO and Be(OH)
Be) of a cosmogenic nuclide precipitates in forms of BeO and Be(OH) attaching with aerosol on the Earth surface. It is accumulated on the sea- and lake-bottoms. Recently, the meteoric
attaching with aerosol on the Earth surface. It is accumulated on the sea- and lake-bottoms. Recently, the meteoric  Be is attracting attention as a powerful tool for investigating the past climate change, because it is affected by the earth- and lorcal- cyclical changes of materials such as atmosphere and water circulation. The
Be is attracting attention as a powerful tool for investigating the past climate change, because it is affected by the earth- and lorcal- cyclical changes of materials such as atmosphere and water circulation. The  Be exists mostly as hydroxide at pH
Be exists mostly as hydroxide at pH  5, and is easy to adhere to soil and mineral surface. Therefore,
5, and is easy to adhere to soil and mineral surface. Therefore,  Be concentration in sediment could be influenced by its grain size composition because fine-grained sediment has a big surface area per unit mass. The purpose of this study is to reveal the relationship between
Be concentration in sediment could be influenced by its grain size composition because fine-grained sediment has a big surface area per unit mass. The purpose of this study is to reveal the relationship between  Be concentrations and the grain-size of river sediments. The samples used were bottom-sediments of 18 rivers flowing into Lake Biwa, Japan. The sediments were sieved to 5 fractions and analyzed each for
Be concentrations and the grain-size of river sediments. The samples used were bottom-sediments of 18 rivers flowing into Lake Biwa, Japan. The sediments were sieved to 5 fractions and analyzed each for  Be concentration by JAEA-AMS-TONO.
Be concentration by JAEA-AMS-TONO.
Shimojo, Kojiro; Fujiwara, Iori*; Fujisawa, Kiyoshi*; Okamura, Hiroyuki; Sugita, Tsuyoshi; Oshima, Tatsuya*; Baba, Yoshinari*; Naganawa, Hirochika
Solvent Extraction Research and Development, Japan, 23(2), p.151 - 159, 2016/05
Liquid-liquid extraction of rare-earth (RE) cations has been investigated using  -dodecyldiglycolamic acid (C
-dodecyldiglycolamic acid (C DGAA) with a secondary amide group, and compared with that using
DGAA) with a secondary amide group, and compared with that using  -dioctyldiglycolamic acid (DODGAA) with a tertiary amide group. C
-dioctyldiglycolamic acid (DODGAA) with a tertiary amide group. C DGAA enables quantitative transfer of all RE cations from moderately acidic solution, while being selective toward the heavier RE cations, and performs better than typical carboxylic-acid-type extractants. However, C
DGAA enables quantitative transfer of all RE cations from moderately acidic solution, while being selective toward the heavier RE cations, and performs better than typical carboxylic-acid-type extractants. However, C DGAA provides low extraction performance and separation ability for RE cations compared with DODGAA because of the weaker basicity of the amide oxygen. Slope analysis demonstrated that RE
DGAA provides low extraction performance and separation ability for RE cations compared with DODGAA because of the weaker basicity of the amide oxygen. Slope analysis demonstrated that RE transfer with C
transfer with C DGAA proceeded through a proton-exchange reaction, forming a 1:3 complex, RE(C
DGAA proceeded through a proton-exchange reaction, forming a 1:3 complex, RE(C DGAA)
DGAA) . Structural characterization by X-ray diffraction revealed that three
. Structural characterization by X-ray diffraction revealed that three  -butyldiglycolamic acid (C
-butyldiglycolamic acid (C DGAA) molecules coordinated to the La
DGAA) molecules coordinated to the La central ion in a tridentate fashion and the La
central ion in a tridentate fashion and the La primary coordination sphere consisted of three oxygen atoms from the amide group, three oxygen atoms from the ether group, and three oxygen atoms from the carboxy group.
primary coordination sphere consisted of three oxygen atoms from the amide group, three oxygen atoms from the ether group, and three oxygen atoms from the carboxy group.
Kasamatsu, Yoshitaka*; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Li, Z.; Ishii, Yasuo; Tome, Hayato*; Sato, Tetsuya; Kikuchi, Takahiro; Nishinaka, Ichiro; et al.
Chemistry Letters, 38(11), p.1084 - 1085, 2009/10
Times Cited Count:15 Percentile:48.93(Chemistry, Multidisciplinary)We report on the characteristic anion-exchange behavior of the superheavy element dubnium (Db) with atomic number Z = 105 in HF/HNO solution at the fluoride ion concentration [F
solution at the fluoride ion concentration [F ] = 0.003 M. The result clearly demonstrates that the fluoro complex formation of Db is significantly different from that of the group-5 homologue Ta in the 6th period of the periodic table while the behavior of Db is similar to that of the lighter homologue Nb in the 5th period.
] = 0.003 M. The result clearly demonstrates that the fluoro complex formation of Db is significantly different from that of the group-5 homologue Ta in the 6th period of the periodic table while the behavior of Db is similar to that of the lighter homologue Nb in the 5th period.
Sano, Hiroyuki*; Fujisawa, Toshiharu*; Kimura, Akihiko*; Inoue, Masaki; Ukai, Shigeharu*; Onuki, Somei*; Okuda, Takanari*; Abe, Fujio*
Proceedings of 2009 International Congress on Advances in Nuclear Power Plants (ICAPP '09) (CD-ROM), p.9308_1 - 9308_5, 2009/05
Corrosion of structural materials is one of the serious problems when lead-bismuth eutectic alloy (LBE) is used as a coolant material in next generation nuclear systems. In this study, dissolution experiments of synthetic Fe-Cr-Al alloys and developed super ODS steel candidates into LBE under several partial pressures of oxygen were conducted. Dissolution behaviors of major components in such steels into LBE were investigated. Interfacial behavior between LBE and steels was also observed. In addition, partial potential diagrams of the Fe-Cr-Al-O system at several conditions were established as basic data. From the potential diagrams, the partial pressure range of oxygen was estimated for the stable protective oxide layer formation at the interface. At lower oxygen partial pressure than the pressure that is enough for the formation of the stable oxide layer, a rough oxide layer was formed at the interface in all samples, and the alloy elements dissolved into LBE through it. On the other hand, at the oxygen partial pressure to form stable oxide layer, a dense and very thin oxide layer was formed especially on the higher aluminum content steel, preventing the alloy dissolution into LBE. From the results, aluminum and chromium content in steel were very important for preventing the corrosion by LBE.
Sano, Hiroyuki*; Fujisawa, Toshiharu*; Furukawa, Tomohiro; Aoto, Kazumi
JNC TY9400 2005-007, 27 Pages, 2005/03
Lead-Bismuth eutectic alloy (LBE) has been considered as a prospective coolant for a fast-breeder reactor, but a corrosion of cooling pipe is anticipated when it is used. In the previous study, the solubility of major metallic elements, such as Fe, Cr and Ni, into LBE was measured under extra low oxygen potential. The interactive effect of those elements on the solubility was also examined. However, it is thought that an oxide layer is formed on the surface of the cooling pipe and influences on the corrosion of the pipe. In this year, the measurement of the solubility of major elements of steel into lead-bismuth alloy through Fe-Cr complex oxide was started. In addition, the thermodynamics of the Pb-Bi-O system at 800 K was investigated and the phase diagram of the system was determined. From the above results, the stability diagram of the Pb-Bi-O system was established.
Sano, Hiroyuki*; Fujisawa, Toshiharu*; Furukawa, Tomohiro; Aoto, Kazumi
JNC TY9400 2004-015, 55 Pages, 2004/03
In this study, solubility of major metallic elements in LBE was measured under extra low oxygen potential.
Sano, Hiroyuki*; Fujisawa, Toshiharu*; Furukawa, Tomohiro; Aoto, Kazumi
JNC TY9400 2003-002, 22 Pages, 2003/03
Lead-Bismuth eutectic alloy(LBE) has been considered as a prospective coolant for a fast-breeder reactor. However a corrosion of the structual material is anticipated when it is used at the similar temperature as sodium coolant. In this study, solubility of major metallic elements in LBE is measured under extra low oxygen potential. The interactive effect of those elements on the solubility is also to be examined. Based on the results of JFY2001, measurements of the solubility of iron in LBE at 1273 K were conducted where the partial pressure of oxygen was controlled by using two equilibrium methods: the oxide equilibrium method and the gas equilibrium method. Analytical condition of oxygen content in LBE was also determined. Several solubility data were obtained. However it was not yet enough to do thermodynamic consideration. From the above results, following subjects were extracted for JFY2003 study. (1)To accumulate the solubility data of iron and oxygen in LBE and to consider them thermodynamically. (2)To obtain the solubility data of the other composition element of stainless steels and to evaluate mutual influence of them.
 under high pressure and high perature
under high pressure and high peratureOtaka, Osamu*; Fukui, Hiroyuki*; Kunisada, Taichi*; Fujisawa, Tomoyuki*; Funakoshi, Kenichi*; Utsumi, Wataru; Irifune, Tetsuo*; Kuroda, Koji*; Kikegawa, Takumi*
Physical Review B, 63(17), p.174108_1 - 174108_8, 2001/05
Times Cited Count:127 Percentile:96.62(Materials Science, Multidisciplinary)no abstracts in English
 O
O into Molten Pb-Bi eutectic
into Molten Pb-Bi eutecticSano, Hiroyuki*; Fujisawa, Toshiharu*; Sagawa, Yosuke*; Furukawa, Tomohiro; Aoto, Kazumi
no journal, ,
no abstracts in English
 solutions using a newly developed on-line experimental system
solutions using a newly developed on-line experimental systemTsukada, Kazuaki; Kasamatsu, Yoshitaka; Asai, Masato; Toyoshima, Atsushi; Ishii, Yasuo; Li, Z.; Kikuchi, Takahiro; Sato, Tetsuya; Nishinaka, Ichiro; Nagame, Yuichiro; et al.
no journal, ,
Anion-exchange chromatographic behavior of element 105, dubnium (Db), produced in the  Cm(
Cm( F,5n)
F,5n) Db reaction is investigated together with the homologues Nb and Ta in HF/HNO
Db reaction is investigated together with the homologues Nb and Ta in HF/HNO mixed media using a newly developed on-line experimental system. The result indicates that the adsorption sequence on the anion-exchange resin is Ta
mixed media using a newly developed on-line experimental system. The result indicates that the adsorption sequence on the anion-exchange resin is Ta Nb
Nb Db, and the fluoride complex formation of Db is expected to be weaker than that of homologues.
Db, and the fluoride complex formation of Db is expected to be weaker than that of homologues.
Okabe, Toshihiro*; Ono, Hiroyuki*; Hatayama, Ichiro*; Waguri, Atsushi*; Sawada, Yuzuru*; Tsugawa, Hidehito*; Ichida, Tadao*; Nakane, Akio*; Sano, Teruo*; Tanaka, Kazuaki*; et al.
no journal, ,
 mixed solution using a new on-line chemical apparatus
mixed solution using a new on-line chemical apparatusTsukada, Kazuaki; Kasamatsu, Yoshitaka*; Asai, Masato; Toyoshima, Atsushi; Ishii, Yasuo; Li, Z.; Kikuchi, Takahiro; Sato, Tetsuya; Nishinaka, Ichiro; Nagame, Yuichiro; et al.
no journal, ,
Anion-exchange chromatographic behavior of element 105, dubnium (Db), produced in the  Cm(
Cm( F,5n) reaction is investigated together with the homologues Nb and Ta in HF/HNO
F,5n) reaction is investigated together with the homologues Nb and Ta in HF/HNO mixed media using a newly developed on-line experimental system. The result indicates that the adsorption sequence on the anion-exchange resin is Ta
mixed media using a newly developed on-line experimental system. The result indicates that the adsorption sequence on the anion-exchange resin is Ta  Nb
Nb  Db, and the fluoride complex formation of Db is expected to be weaker than that of homologues.
Db, and the fluoride complex formation of Db is expected to be weaker than that of homologues.
 mixed solution
mixed solutionKasamatsu, Yoshitaka*; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Li, Z.; Ishii, Yasuo; Sato, Tetsuya; Nishinaka, Ichiro; Kikuchi, Takahiro; Haba, Hiromitsu*; et al.
no journal, ,
Dubnium-262 was produced in the  Cm(
Cm( F, 5n) reaction at the JAEA tandem accelerator. Reaction products were rapidly transported by a He/KF gas-jet system to the chemistry laboratory. Anion-exchange behavior of Db in HF/HNO
F, 5n) reaction at the JAEA tandem accelerator. Reaction products were rapidly transported by a He/KF gas-jet system to the chemistry laboratory. Anion-exchange behavior of Db in HF/HNO solution was then investigated using the newly developed on-line ion-exchange and
solution was then investigated using the newly developed on-line ion-exchange and  -particle detection apparatus. Distribution coefficients of Db were clearly smaller than those of Ta, the closest homologue in the periodic table, and were close to those of lighter homologue Nb and also the pseudo homologue Pa. Fluoride complex formation of Db will be discussed.
-particle detection apparatus. Distribution coefficients of Db were clearly smaller than those of Ta, the closest homologue in the periodic table, and were close to those of lighter homologue Nb and also the pseudo homologue Pa. Fluoride complex formation of Db will be discussed.
 mixed solution using an on-line chemical apparatus
mixed solution using an on-line chemical apparatusTsukada, Kazuaki; Kasamatsu, Yoshitaka*; Asai, Masato; Toyoshima, Atsushi; Ishii, Yasuo; Li, Z.; Kikuchi, Takahiro; Sato, Tetsuya; Nagame, Yuichiro; Sch del, M.; et al.
del, M.; et al.
no journal, ,
We have investigated the chemical behavior of Db together with its group-5 homologues by anion-exchange chromatography in HF/HNO mixed solution using a rapid online chemical apparatus (AIDA-II). The nuclides
mixed solution using a rapid online chemical apparatus (AIDA-II). The nuclides  Db,
Db,  Nb and
Nb and 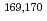 Ta were produced in the
Ta were produced in the  Cm(
Cm( F,
F, 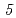 n),
n), 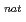 Ge(
Ge( F,
F,  n) and
n) and 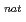 Gd(
Gd( F,
F,  n) reactions, respectively, at the JAEA tandem accelerator. On-line anion-exchange separations of Db, Nb and Ta were performed using the AIDA-II. Thousand times of anion-exchange separations were conducted using AIDA-II.
n) reactions, respectively, at the JAEA tandem accelerator. On-line anion-exchange separations of Db, Nb and Ta were performed using the AIDA-II. Thousand times of anion-exchange separations were conducted using AIDA-II.  events were registered, and the
events were registered, and the 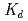 values for Db was evaluated. From these results, the adsorption sequence Pa
values for Db was evaluated. From these results, the adsorption sequence Pa  Db
Db  Nb
Nb 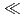 Ta was determined. The present result shows a notable difference in the adsorption behavior between Db and its homologue Ta. In the conference, the present status and the perspective of the aqueous chemistry of Db at JAEA will be also presented.
Ta was determined. The present result shows a notable difference in the adsorption behavior between Db and its homologue Ta. In the conference, the present status and the perspective of the aqueous chemistry of Db at JAEA will be also presented.
Takayama, Reona*; Oe, Kazuhiro*; Komori, Yukiko*; Fujisawa, Hiroyuki*; Kuriyama, Ai*; Kikutani, Yuki*; Kikunaga, Hidetoshi*; Kasamatsu, Yoshitaka*; Yoshimura, Takashi*; Takahashi, Naruto*; et al.
no journal, ,
The extraction behavior of trivalent actinides (Am, Cm, Cf, Es, and Fm) into di(2-ethylhexyl)phosphoric acid, HDEHP, in benzene from HNO was studied together with lanthanides. The extraction constants,
was studied together with lanthanides. The extraction constants, 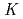
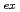 values, of lanthanides, Am, Cm, Cf, and Es were determined by a batch method using stable lanthanide isotopes and radiotracers of
values, of lanthanides, Am, Cm, Cf, and Es were determined by a batch method using stable lanthanide isotopes and radiotracers of  Am,
Am,  Cm,
Cm,  Cf, and
Cf, and 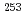 Es, respectively, while that of Fm was measured with
Es, respectively, while that of Fm was measured with 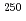 Fm which was produced in the
Fm which was produced in the  U(
U(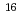 O,4n) reaction and was on-line supplied by a gas-jet method at the AVF cyclotron of RCNP, Osaka University. The
O,4n) reaction and was on-line supplied by a gas-jet method at the AVF cyclotron of RCNP, Osaka University. The 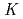
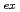 values were determined by the slope analysis for the variation of the distribution ratio as a function of the concentration of HDEHP. We found that the
values were determined by the slope analysis for the variation of the distribution ratio as a function of the concentration of HDEHP. We found that the 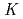
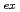 of Fm is smaller than that of Dy in spite of the similar ionic radii while those of other actinides are almost the same as those of each lanthanide with a similar ionic radius.
of Fm is smaller than that of Dy in spite of the similar ionic radii while those of other actinides are almost the same as those of each lanthanide with a similar ionic radius.
Kobayashi, Yuichi*; Fujisawa, Yasuo*; Yoshino, Hiroyuki*; Sugita, Yutaka; Kawaguchi, Tatsuya; Hatanaka, Koichiro; Shibata, Masahiro; Yamamura, Masato*; Kamisakoda, Kazuto*; Shimbo, Hiroshi*; et al.
no journal, ,
This paper reports on the development of ISRE (the Integrated System for Repository Engineering), as a knowledge management tool on repository design and engineering technology, which manages and inherits the information and knowledge of each phase of the disposal project of radioactive waste lasting around 100 years, and which also enables reasonable design of the repository by using accumulated and integrated information. The content of this paper is a formulation of the integrated model for the ISRE and management method of the attribute information associated with the data model.
Shimbo, Hiroshi*; Yamamura, Masato*; Kamisakoda, Kazuto*; Sugita, Yutaka; Kawaguchi, Tatsuya; Hatanaka, Koichiro; Shibata, Masahiro; Fujisawa, Yasuo*; Yoshino, Hiroyuki*; Kobayashi, Yuichi*; et al.
no journal, ,
This paper reports on the development of ISRE (the Integrated System for Repository Engineering), as a knowledge management tool on repository design and engineering technology, which manages and inherits the information and knowledge of each phase of the disposal project of radioactive waste lasting around 100 years, and which also enables reasonable design of the repository by using accumulated and integrated information. The content of this paper is the utilization of the ISRE in the disposal project.
Yoshino, Hiroyuki*; Fujisawa, Yasuo*; Kobayashi, Yuichi*; Sugita, Yutaka; Kageyama, Takeshi; Hane, Koji*; Sahara, Fumihiro*; Yabuki, Nobuyoshi*; Makanae, Koji*
no journal, ,
This paper reports development of ISRE (the Integrated System for Repository Engineering), as a knowledge management tool on the repository design and engineering technology, which manages and inherits the information and knowledge of each phase of the disposal project of the radioactive waste during around 100 years, which also enables reasonable design of the repository by using accumulated and integrated information. Content of this paper is current status of the prototype of the iSRE.
Hane, Koji*; Sahara, Fumihiro*; Sugita, Yutaka; Kageyama, Takeshi; Fujisawa, Yasuo*; Yoshino, Hiroyuki*; Kobayashi, Yuichi*; Yabuki, Nobuyoshi*; Makanae, Koji*
no journal, ,
This paper reports development of ISRE (the Integrated System for Repository Engineering), as a knowledge management tool on the repository design and engineering technology, which manages and inherits the information and knowledge of each phase of the disposal project of the radioactive waste during around 100 years, which also enables reasonable design of the repository by using accumulated and integrated information. Content of this paper is outline of trial utilization of the prototype of the iSRE and the extracted issues.