Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Shiraishi, Yuta*; Matsuya, Yusuke; Kusumoto, Tamon*; Fukunaga, Hisanori*
Physics in Medicine & Biology, 69(1), p.015017_1 - 015017_14, 2024/01
Times Cited Count:0 Percentile:0.05(Engineering, Biomedical)FLASH radiotherapy (FLASH-RT) using ultra-high dose rate ( 40 Gy/sec) is known as a new treatment which is expected to enable preserving normal tissue functions, compared to the conventional radiotherapy (CONV-RT) with high dose rate (
40 Gy/sec) is known as a new treatment which is expected to enable preserving normal tissue functions, compared to the conventional radiotherapy (CONV-RT) with high dose rate (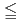 6 Gy/min). To date, it is believed that the modulation of chemical processes caused by interactions between radiation tracks under FLASH-RT is a key factor in the functional preservation of normal tissues; however, the relationship between changes in chemical processes and cellular responses remains uncertain. In this study, we developed a prediction model (integrated microdosimetric-kinetic (IMK) model for FLASH-RT) taking into account of the relationship between the chemical process and the DNA damage yields (which is the initial response) under ultra-high dose rate irradiation, to investigate the cellular mechanisms. As a result, the developed model considering the chemical-processes dependent change in DNA damage yields successfully reproduced the measured cell-killing effects of both CONV-RT and FLASH-RT for various cell line types. This model development would contribute on not only precisely understanding of cellular mechanisms after FLASH-RT irradiation but also enabling the prediction of therapeutic effects with high precision.
6 Gy/min). To date, it is believed that the modulation of chemical processes caused by interactions between radiation tracks under FLASH-RT is a key factor in the functional preservation of normal tissues; however, the relationship between changes in chemical processes and cellular responses remains uncertain. In this study, we developed a prediction model (integrated microdosimetric-kinetic (IMK) model for FLASH-RT) taking into account of the relationship between the chemical process and the DNA damage yields (which is the initial response) under ultra-high dose rate irradiation, to investigate the cellular mechanisms. As a result, the developed model considering the chemical-processes dependent change in DNA damage yields successfully reproduced the measured cell-killing effects of both CONV-RT and FLASH-RT for various cell line types. This model development would contribute on not only precisely understanding of cellular mechanisms after FLASH-RT irradiation but also enabling the prediction of therapeutic effects with high precision.
 -H2AX focus formation assay based on track-structure simulation
-H2AX focus formation assay based on track-structure simulationYachi, Yoshie*; Matsuya, Yusuke*; Yoshii, Yuji*; Fukunaga, Hisanori*; Date, Hiroyuki*; Kai, Takeshi
International Journal of Molecular Sciences (Internet), 24(2), p.1386_1 - 1386_14, 2023/01
Times Cited Count:2 Percentile:75.46(Biochemistry & Molecular Biology)When living cells are irradiated with radiation and complex damage is formed within a few nanometers of DNA, it is believed to induce biological effects such as cell death. In general, complex DNA damage formed in cells can be detected experimentally by fluorescence microscopy, because the area around the damage site emits light like a focus point when a fluorophore is used. However, this detection method has not been able to analyze the degree of complexity of DNA damage. Therefore, in this study, we addressed on the measured focus size and evaluated the degree of complexity of DNA damage using a track structure analysis code. As a result, we found that as DNA damage becomes more complex, the focus size also increases. Our findings are expected to provide a new analytical method for elucidating the initial factors of radiation biological effects.
Matsuya, Yusuke; Kusumoto, Tamon*; Yachi, Yoshie*; Hirata, Yuho; Miwa, Misako*; Ishikawa, Masayori*; Date, Hiroyuki*; Iwamoto, Yosuke; Matsuyama, Shigeo*; Fukunaga, Hisanori*
AIP Advances (Internet), 12(2), p.025013_1 - 025013_9, 2022/02
Times Cited Count:4 Percentile:59.24(Nanoscience & Nanotechnology)Boron Neutron Capture Therapy (BNCT) is a radiation therapy, which can selectively eradicate solid tumors by  -particles and Li ions generated through the nuclear reaction between thermal neutron and
-particles and Li ions generated through the nuclear reaction between thermal neutron and  B in tumor cells. With the development of accelerator-based neutron sources that can be installed in medical institutions, accelerator-based boron neutron capture therapy is expected to become available at several medical institutes around the world in the near future. Lithium is one of the targets that can produce thermal neutrons from the
B in tumor cells. With the development of accelerator-based neutron sources that can be installed in medical institutions, accelerator-based boron neutron capture therapy is expected to become available at several medical institutes around the world in the near future. Lithium is one of the targets that can produce thermal neutrons from the  Li(p,n)
Li(p,n) Be near-threshold reaction. Particle and Heavy Ion Transport code System (PHITS) is a general-purpose Monte Carlo code, which can simulate a variety of diverse particle types and nuclear reactions. The latest PHITS code enables simulating the generation of neutrons from the
Be near-threshold reaction. Particle and Heavy Ion Transport code System (PHITS) is a general-purpose Monte Carlo code, which can simulate a variety of diverse particle types and nuclear reactions. The latest PHITS code enables simulating the generation of neutrons from the  Li(p,n)
Li(p,n) Be reactions by using Japanese Evaluated Nuclear Data Library (JENDL-4.0/HE). In this study, we evaluated the neutron fluence using the PHITS code by comparing it to reference data. The subsequent neutron transport simulations were also performed to evaluate the boron trifluoride (BF
Be reactions by using Japanese Evaluated Nuclear Data Library (JENDL-4.0/HE). In this study, we evaluated the neutron fluence using the PHITS code by comparing it to reference data. The subsequent neutron transport simulations were also performed to evaluate the boron trifluoride (BF ) detector responses and the recoiled proton fluence detected by a CR-39 plastic detector. As a result, these comparative studies confirmed that the PHITS code can accurately simulate neutrons generated from an accelerator using a Li target. The PHITS code has a significant potential for contributing to more precise evaluating accelerator-based neutron fields and understandings of therapeutic effects of BNCT.
) detector responses and the recoiled proton fluence detected by a CR-39 plastic detector. As a result, these comparative studies confirmed that the PHITS code can accurately simulate neutrons generated from an accelerator using a Li target. The PHITS code has a significant potential for contributing to more precise evaluating accelerator-based neutron fields and understandings of therapeutic effects of BNCT.
Fukunaga, Hisanori*; Matsuya, Yusuke
Hoshasen Seibutsu Kenkyu, 56(2), p.208 - 223, 2021/06
Boron Neutron Capture Therapy (BNCT) is one of the radiation therapies, enabling selectively eradicating tumors by short-range a-particles and Li ions generated through the nuclear reaction between thermal neutron and  B within tumor cells. With the development of the accelerator-based neutron source in the recent decades, it is expected that BNCT will be available in many medical facilities worldwide in the future. BNCT irradiation needs a relatively long dose-delivery time after taking up boron drug into tumor cells by intravenous injection. During the period, it is suspected that the boron drug is heterogeneously taken up into cells and its concentration changes continuously, leading to the modification of curative effects from the pharmacological and biological viewpoints. However, the model development for precisely predicting curative effects after BNCT irradiation is still ongoing. Here, we introduce the forefront of model development for estimating the curative effects during BNCT irradiation with high accuracy. This review can create the synergetic effects through an interdisciplinary research approach that can connect the fields of physics, pharmacology, biology and medicine, and would pave the way for new era of BNCT.
B within tumor cells. With the development of the accelerator-based neutron source in the recent decades, it is expected that BNCT will be available in many medical facilities worldwide in the future. BNCT irradiation needs a relatively long dose-delivery time after taking up boron drug into tumor cells by intravenous injection. During the period, it is suspected that the boron drug is heterogeneously taken up into cells and its concentration changes continuously, leading to the modification of curative effects from the pharmacological and biological viewpoints. However, the model development for precisely predicting curative effects after BNCT irradiation is still ongoing. Here, we introduce the forefront of model development for estimating the curative effects during BNCT irradiation with high accuracy. This review can create the synergetic effects through an interdisciplinary research approach that can connect the fields of physics, pharmacology, biology and medicine, and would pave the way for new era of BNCT.
Fukunaga, Hisanori*; Matsuya, Yusuke; Tokuue, Koichi*; Omura, Motoko*
British Journal of Radiology, 93(1111), p.20200311_1 - 20200311_4, 2020/07
Times Cited Count:3 Percentile:73.58(Radiology, Nuclear Medicine & Medical Imaging)Boron neutron capture therapy (BNCT) has attracted attention as a selective treatment approach for cancer cells while sparing surrounding normal cells. The basic concept of BNCT was developed in the 1930s, but it has not yet been commonly popular in clinical practice, even though there is now a large number of experimental and translational studies demonstrating its marked therapeutic potential. With the development of neutron accelerators that can be installed in medical institutions, accelerator-based BNCT is expected to become available at several medical institutes around the world in the near future. In this commentary, from the point of view of microdosimetry, we discuss the biological effects of BNCT, especially the underlying mechanisms of cell responses. The recent development of new treatment methods that combine proton beam sources and BNCT technology is expected to contribute significantly improving the prognosis of cancer treatment in the near future. Therefore, radiobiologists in the field of BNCT and related techniques will have a significant role to play in creating synergy effects in clinical oncology.
Matsuya, Yusuke; Fukunaga, Hisanori*; Omura, Motoko*; Date, Hiroyuki*
Cells, 9(5), p.1117_1 - 1117_16, 2020/05
Times Cited Count:20 Percentile:69.88(Cell Biology)When delivering a high absorbed dose to cancer cells following boron neutron capture therapy (BNCT), heterogeneous dose distribution, the time line of  B concentrations and the long dose-delivery time must be considered. Changes in radiosensitivity during such a long dose-delivery time can reduce the probability of tumor control; however, such change has not yet been evaluated. Here, we developed a cell-killing model that accounts for changes in microdosimetric quantities and dose rates depending on the
B concentrations and the long dose-delivery time must be considered. Changes in radiosensitivity during such a long dose-delivery time can reduce the probability of tumor control; however, such change has not yet been evaluated. Here, we developed a cell-killing model that accounts for changes in microdosimetric quantities and dose rates depending on the  B concentration and investigated dose-rate effects (cell recovery during BNCT irradiation) of melanoma. The developed model shows good agreement with in-vitro experimental survival data for exposure to
B concentration and investigated dose-rate effects (cell recovery during BNCT irradiation) of melanoma. The developed model shows good agreement with in-vitro experimental survival data for exposure to  Co
Co  -rays, thermal neutrons, and BNCT. The model estimation suggests that the impact of cell recovery during BNCT irradiations with high linear energy transfer (LET) is reduced compared to
-rays, thermal neutrons, and BNCT. The model estimation suggests that the impact of cell recovery during BNCT irradiations with high linear energy transfer (LET) is reduced compared to  Co
Co  -rays irradiation with low LET. The present model is expected to predict radio-sensitivity for BNCT irradiations.
-rays irradiation with low LET. The present model is expected to predict radio-sensitivity for BNCT irradiations.
Fukunaga, Hisanori*; Yokoya, Akinari
Journal of Radiation Research, 57(1), p.98 - 100, 2016/01
Times Cited Count:8 Percentile:85.66(Biology)Matsuya, Yusuke; Omura, Motoko*; Fukunaga, Hisanori*
no journal, ,
Boron neutron capture therapy (BNCT) is a treatment method enabling selectively eradicating tumors by  -particles and Li ions generated through the nuclear reaction between neutrons and
-particles and Li ions generated through the nuclear reaction between neutrons and  B within tumor cells. While the accelerator-based neutron source has been developed, the intracellular
B within tumor cells. While the accelerator-based neutron source has been developed, the intracellular  B concentration dynamically changes after intravenous injection of the boron into patients, and the neutron-delivery time is relatively long for 30 minutes or more. However, the quantitative analysis for investigating the impacts of DNA repair during irradiation on curative effects is insufficient. In this study, we developed a mathematical model for predicting the therapeutic effects that considers the change in intracellular
B concentration dynamically changes after intravenous injection of the boron into patients, and the neutron-delivery time is relatively long for 30 minutes or more. However, the quantitative analysis for investigating the impacts of DNA repair during irradiation on curative effects is insufficient. In this study, we developed a mathematical model for predicting the therapeutic effects that considers the change in intracellular  B concentration and DNA repair, and performed detailed analysis. The model developed in this study considers the following two factors: one is microdosimetric quantities (physical characteristics) that depends on
B concentration and DNA repair, and performed detailed analysis. The model developed in this study considers the following two factors: one is microdosimetric quantities (physical characteristics) that depends on  B concentration, and the other is DNA repair dynamics (biological process) during irradiation. The model estimation results exhibits that the importance of DNA repair during irradiation is reduced in the case of BNCT with a higher dose at sub-cellular scale than photons. It was also suggested that the DNA repair effects on tumor control cannot be ignored even in the case of BNCT. In the future, further accumulation of radiobiological data is desired for improving models.
B concentration, and the other is DNA repair dynamics (biological process) during irradiation. The model estimation results exhibits that the importance of DNA repair during irradiation is reduced in the case of BNCT with a higher dose at sub-cellular scale than photons. It was also suggested that the DNA repair effects on tumor control cannot be ignored even in the case of BNCT. In the future, further accumulation of radiobiological data is desired for improving models.
Fukunaga, Hisanori*; Matsuya, Yusuke; Yachi, Yoshie*; Date, Hiroyuki*
no journal, ,
Boron neutron capture therapy (BNCT) is a radiotherapy which enables eradicating tumor by the dose concentration on tumor cells by  rays and Li ions generated from the nuclear reaction between thermal neutrons and boron. With the development of accelerator-based neutron sources and the Japanese insurance listing, it is expected that accelerator-based BNCT will be installed in many medical institutions in the future. In the accelerator-based BNCT, boron-containing compounds, i.e., BPA, are administered by intravenous injection and are taken up into tumor cells, so it is expected that cellular BPA concentration becomes spatially heterogeneous. However, the effects of such heterogenous BPA concentration on the therapeutic effects remains unclear. In this study, we measured the heterogenous distribution of BPA in tumor cells (cell cycle dependence) using CR-39 detector. As a result, the number of reactions per unit area in the BPA-administered cells was increased by about 20% in S/G
rays and Li ions generated from the nuclear reaction between thermal neutrons and boron. With the development of accelerator-based neutron sources and the Japanese insurance listing, it is expected that accelerator-based BNCT will be installed in many medical institutions in the future. In the accelerator-based BNCT, boron-containing compounds, i.e., BPA, are administered by intravenous injection and are taken up into tumor cells, so it is expected that cellular BPA concentration becomes spatially heterogeneous. However, the effects of such heterogenous BPA concentration on the therapeutic effects remains unclear. In this study, we measured the heterogenous distribution of BPA in tumor cells (cell cycle dependence) using CR-39 detector. As a result, the number of reactions per unit area in the BPA-administered cells was increased by about 20% in S/G /M phase cells compared to that in G
/M phase cells compared to that in G /S phase. This outcome revealed the cell-cycle dependence on the intercellular BPA concentration and the boron dose, suggesting that such heterogeneity might affect the curative effects for BNCT.
/S phase. This outcome revealed the cell-cycle dependence on the intercellular BPA concentration and the boron dose, suggesting that such heterogeneity might affect the curative effects for BNCT.