Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
He, X.*; Kagi, Hiroyuki*; Komatsu, Kazuki*; Iizuka, Riko*; Okajima, Hajime*; Hattori, Takanori; Sano, Asami; Machida, Shinichi*; Abe, Jun*; Goto, Hirotada*; et al.
Journal of Molecular Structure, 1310, p.138271_1 - 138271_8, 2024/08
High-pressure responses of the O-D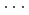 F hydrogen bonds in deuterated magnesium hydroxyfluoride were investigated using neutron powder diffraction and Raman spectroscopy. The Rietveld analysis at ambient conditions revealed a chemical formula of Mg(OD)
F hydrogen bonds in deuterated magnesium hydroxyfluoride were investigated using neutron powder diffraction and Raman spectroscopy. The Rietveld analysis at ambient conditions revealed a chemical formula of Mg(OD)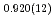 F
F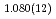 and hydroxyl group/fluorine disorder (OD/F disorder) in the crystal structure, which gave rise to two hydrogen-bonding configurations. The Rietveld analysis showed the hydrogen-bonding geometries remains up to 9.8 GPa, indicating no pressure-induced strengthening of hydrogen bonds. The Raman spectra at ambient conditions showed three hydroxyl stretching bands at 2613, 2694, and 2718 cm
and hydroxyl group/fluorine disorder (OD/F disorder) in the crystal structure, which gave rise to two hydrogen-bonding configurations. The Rietveld analysis showed the hydrogen-bonding geometries remains up to 9.8 GPa, indicating no pressure-induced strengthening of hydrogen bonds. The Raman spectra at ambient conditions showed three hydroxyl stretching bands at 2613, 2694, and 2718 cm . The high frequencies of the O-D stretching modes indicated that the hydroxyls should be involved in weak or none hydrogen-bonding interactions. Up to 20.2 GPa, the mode initially centered at 2694 cm
. The high frequencies of the O-D stretching modes indicated that the hydroxyls should be involved in weak or none hydrogen-bonding interactions. Up to 20.2 GPa, the mode initially centered at 2694 cm displayed a pressure-induced blue shift, revealing no strengthening of hydrogen bonds under compression. We discuss the existence of hydrogen bonds and the causes of the blue-shifting hydroxyls at ambient and at high pressures.
displayed a pressure-induced blue shift, revealing no strengthening of hydrogen bonds under compression. We discuss the existence of hydrogen bonds and the causes of the blue-shifting hydroxyls at ambient and at high pressures.
Hattori, Takanori; Suzuki, Koji*; Miyo, Tatsuya*; Ito, Takayoshi*; Machida, Shinichi*
Nuclear Instruments and Methods in Physics Research A, 1059, p.168956_1 - 168956_9, 2024/02
Times Cited Count:0 Percentile:0.02(Instruments & Instrumentation)Radial collimators (RC) with a 0.5 mm gauge size (GS) were specially designed for high-pressure neutron diffraction experiments and their performance and efficacy were investigated. The RCs with nominal GS of 0.75 mm, 1.5 mm, and 3.0 mm effectively exhibited GS of 0.50 mm, 1.07 mm, and 2.78 mm, respectively. The transmissions of all three RCs were almost equivalent. The assessment using a P-E press and a DAC revealed that the anvil scattering was considerably minimized and the sample-to-anvil signal ratio reached values of 0.5 and 2.0 for the PE press and DAC, respectively, when using the 0.5 mm-GS RCs. These results indicate that the 0.5mm-GS RCs have been fabricated as intended and exhibit efficacy for the high-pressure-neutron diffraction experiments, specifically those exceeding 30 GPa. Among those ever manufactured for neutron scattering experiments, the RCs display the smallest GS.
 structure transition under high pressure
structure transition under high pressureYamanaka, Takamitsu*; Nakamoto, Yuki*; Sakata, Masafumi*; Shimizu, Katsuya*; Hattori, Takanori
Physics and Chemistry of Minerals, 51(1), p.4_1 - 4_10, 2024/02
Times Cited Count:0 Percentile:0.34(Materials Science, Multidisciplinary)Neutron and synchrotron X-ray diffraction and electric conductivity measurements of FeTiO ilmenite were performed under pressures. Ilmenite structure is retained up to 28 GPa. Structure analysis revealed that FeO
ilmenite were performed under pressures. Ilmenite structure is retained up to 28 GPa. Structure analysis revealed that FeO and TiO
and TiO are compressible and less compressible below 8 GPa, respectively. The resistivity is lowest along the Fe-Ti direction that has shortest interatomic distance among all the metal ion pairs. The resistivity in the direction normal to c-axis monotonically decreases with pressure, whereas that along c-axis shows hallow-shape with pressure. Maximum entropy analysis shows that electron configuration of Fe
are compressible and less compressible below 8 GPa, respectively. The resistivity is lowest along the Fe-Ti direction that has shortest interatomic distance among all the metal ion pairs. The resistivity in the direction normal to c-axis monotonically decreases with pressure, whereas that along c-axis shows hallow-shape with pressure. Maximum entropy analysis shows that electron configuration of Fe (3
(3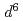 ) is more strongly changed than Ti
) is more strongly changed than Ti (3
(3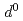 ) under compression. The anisotropic electrical conductivity and non-uniform structure change of Fe-Ti interatomic distance can be explained by the possible spin transition from high-spin state to intermediate-spin state of Fe cation.
) under compression. The anisotropic electrical conductivity and non-uniform structure change of Fe-Ti interatomic distance can be explained by the possible spin transition from high-spin state to intermediate-spin state of Fe cation.
 neutron diffraction
neutron diffractionKobayashi, Hiroki*; Komatsu, Kazuki*; Ito, Hayate*; Machida, Shinichi*; Hattori, Takanori; Kagi, Hiroyuki*
Journal of Physical Chemistry Letters (Internet), 14(47), p.10664 - 10669, 2023/11
Times Cited Count:0 Percentile:0.01(Chemistry, Physical)Ice IV is a metastable high-pressure phase of ice in which the water molecules exhibit orientational disorder. Although orientational ordering is commonly observed for other ice phases, it has not been reported for ice IV. We conducted  powder neutron diffraction experiments for DCl-doped D
powder neutron diffraction experiments for DCl-doped D O ice IV to investigate hydrogen ordering in ice IV. We found abrupt changes in the temperature derivative of unit cell volume, dV/dT, at about 120 K, and revealed their slightly ordered structure at low temperatures based on the Rietveld method. The occupancy of the D1 site deviates from 0.5; it increased when samples were cooled at higher pressures and reached 0.282(5) at 2.38 GPa, 58 K. Our results evidence the presence of a low-symmetry hydrogen-ordered state corresponding to ice IV. It seems, however, difficult to experimentally access the completely ordered phase corresponding to ice IV by slow cooling at high pressure.
O ice IV to investigate hydrogen ordering in ice IV. We found abrupt changes in the temperature derivative of unit cell volume, dV/dT, at about 120 K, and revealed their slightly ordered structure at low temperatures based on the Rietveld method. The occupancy of the D1 site deviates from 0.5; it increased when samples were cooled at higher pressures and reached 0.282(5) at 2.38 GPa, 58 K. Our results evidence the presence of a low-symmetry hydrogen-ordered state corresponding to ice IV. It seems, however, difficult to experimentally access the completely ordered phase corresponding to ice IV by slow cooling at high pressure.
Yamashita, Keishiro*; Nakayama, Kazuya*; Komatsu, Kazuki*; Ohara, Takashi; Munakata, Koji*; Hattori, Takanori; Sano, Asami; Kagi, Hiroyuki*
Acta Crystallographica Section B; Structural Science, Crystal Engineering and Materials (Internet), 79(5), p.414 - 426, 2023/10
Times Cited Count:0 Percentile:0.02(Chemistry, Multidisciplinary)The structure of a recently-found hyperhydrated form of sodium chloride, NaCl 13H(D)
13H(D) O, has been determined by
O, has been determined by  single-crystal neutron diffraction at 1.7 GPa and 298 K. It has large hydrogen-bond networks and some water molecules have distorted bonding features such as bifurcated hydrogen bonds and five-coordinated water molecules. The hydrogen-bond network has similarities to ice VI in terms of network topology and disordered hydrogen bonds. Assuming the equivalence of network components connected by pseudo symmetries, the overall network structure of this hydrate can be expressed by breaking it down into smaller structural units which correspond to the ice VI network structure. This hydrogen-bond network contains orientational disorder of water molecules in contrast to the known salt hydrates. Here, we present an example for further insights into a hydrogen-bond network containing ionic species.
single-crystal neutron diffraction at 1.7 GPa and 298 K. It has large hydrogen-bond networks and some water molecules have distorted bonding features such as bifurcated hydrogen bonds and five-coordinated water molecules. The hydrogen-bond network has similarities to ice VI in terms of network topology and disordered hydrogen bonds. Assuming the equivalence of network components connected by pseudo symmetries, the overall network structure of this hydrate can be expressed by breaking it down into smaller structural units which correspond to the ice VI network structure. This hydrogen-bond network contains orientational disorder of water molecules in contrast to the known salt hydrates. Here, we present an example for further insights into a hydrogen-bond network containing ionic species.
 solution in the gigapascal pressure range
solution in the gigapascal pressure rangeYamaguchi, Toshio*; Fukuyama, Nami*; Yoshida, Koji*; Katayama, Yoshinori*; Machida, Shinichi*; Hattori, Takanori
Liquids, 3(3), p.288 - 302, 2023/09
We report the structure of an aqueous 2 mol/kg MgCl solution at pressures from 0.1 MPa to 4 GPa and temperatures from 300 to 500 K revealed by X-ray and neutron scattering measurements. The scattering data are analyzed by empirical potential structure refinement (EPSR) modeling to derive the pair distribution functions, coordination number distributions, angle distributions, and spatial density functions as a function of pressure and temperature. Mg
solution at pressures from 0.1 MPa to 4 GPa and temperatures from 300 to 500 K revealed by X-ray and neutron scattering measurements. The scattering data are analyzed by empirical potential structure refinement (EPSR) modeling to derive the pair distribution functions, coordination number distributions, angle distributions, and spatial density functions as a function of pressure and temperature. Mg forms rigid solvation shells extended to the third shell; the first solvation shell of six-fold octahedral coordination with about six water molecules at 0 GPa transforms into about five water molecules and one Cl
forms rigid solvation shells extended to the third shell; the first solvation shell of six-fold octahedral coordination with about six water molecules at 0 GPa transforms into about five water molecules and one Cl due to the formation of the contact ion pairs in the GPa pressure range. The Cl
due to the formation of the contact ion pairs in the GPa pressure range. The Cl solvation shows a substantial pressure dependence; the coordination number of a water oxygen atom around Cl
solvation shows a substantial pressure dependence; the coordination number of a water oxygen atom around Cl increases from 8 at 0.1 MPa/300 K to 10 at 4 GPa/500 K. The solvent water transforms the tetrahedral network structure at 0.1 MPa/300 K to a densely packed structure in the GPa pressure range; the number of water oxygen atoms around a central water molecule gradually increases from 4.6 at 0.1 MPa/298 K to 8.4 at 4 GPa/500 K.
increases from 8 at 0.1 MPa/300 K to 10 at 4 GPa/500 K. The solvent water transforms the tetrahedral network structure at 0.1 MPa/300 K to a densely packed structure in the GPa pressure range; the number of water oxygen atoms around a central water molecule gradually increases from 4.6 at 0.1 MPa/298 K to 8.4 at 4 GPa/500 K.
 ; Bandgap narrowing, metallization, and remarkable enhancement of photoelectric activity
; Bandgap narrowing, metallization, and remarkable enhancement of photoelectric activityFang, Y.*; Kong, L.*; Wang, R.*; Zhang, Z.*; Li, Z.*; Wu, Y.*; Bu, K.*; Liu, X.*; Yan, S.*; Hattori, Takanori; et al.
Materials Today Physics (Internet), 34, p.101083_1 - 101083_7, 2023/05
Times Cited Count:1 Percentile:0(Materials Science, Multidisciplinary)The layered van der Waals halides are particularly sensitive to external pressure, suggesting a feasible route to pinpoint their structure with extraordinary behavior. However, a very sensitive pressure response usually lead to a detrimental phase transition and/or lattice distortion, making the approach of materials manipulation in a continuous manner remain challenging. Here, the extremely weak interlayer coupling and high tunability of layered RhI crystals are observed. A pressure-driven phase transition occurs at a moderate pressure of 5 GPa, interlinking to a change of layer stack mode. Strikingly, such a phase transition does not affect the tendency of quasi-linear bandgap narrowing, and a metallization with an ultra-broad tunability of 1.3 eV redshift is observed at higher pressures. Moreover, the carrier concentration increases by 4 orders of magnitude at 30 GPa, and the photocurrent enhances by 5 orders of magnitude at 7.8 GPa. These findings create new opportunities for exploring, tuning, and understanding the van der Waals halides by harnessing their unusual feature of a layered structure, which is promising for future devices based on materials-by-design that are atomically thin.
crystals are observed. A pressure-driven phase transition occurs at a moderate pressure of 5 GPa, interlinking to a change of layer stack mode. Strikingly, such a phase transition does not affect the tendency of quasi-linear bandgap narrowing, and a metallization with an ultra-broad tunability of 1.3 eV redshift is observed at higher pressures. Moreover, the carrier concentration increases by 4 orders of magnitude at 30 GPa, and the photocurrent enhances by 5 orders of magnitude at 7.8 GPa. These findings create new opportunities for exploring, tuning, and understanding the van der Waals halides by harnessing their unusual feature of a layered structure, which is promising for future devices based on materials-by-design that are atomically thin.
 Ni
Ni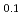 D
D at high
at high 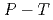 conditions
conditionsShito, Chikara*; Kagi, Hiroyuki*; Kakizawa, Sho*; Aoki, Katsutoshi*; Komatsu, Kazuki*; Iizuka, Riko*; Abe, Jun*; Saito, Hiroyuki*; Sano, Asami; Hattori, Takanori
American Mineralogist, 108(4), p.659 - 666, 2023/04
Times Cited Count:1 Percentile:64.83(Geochemistry & Geophysics)The phase relation and crystal structure of Fe Ni
Ni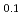 H
H (D
(D ) at high pressures and temperatures up to 12 GPa and 1000 K were clarified by in-situ X-ray and neutron diffraction measurements. Under
) at high pressures and temperatures up to 12 GPa and 1000 K were clarified by in-situ X-ray and neutron diffraction measurements. Under 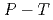 conditions of the present study, no deuterium atoms occupied tetragonal (
conditions of the present study, no deuterium atoms occupied tetragonal ( ) sites of face-centered cubic (fcc) Fe
) sites of face-centered cubic (fcc) Fe Ni
Ni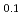 D
D unlike fcc FeH
unlike fcc FeH (D
(D ). The deuterium-induced volume expansion per deuterium
). The deuterium-induced volume expansion per deuterium 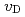 was determined as 2.45(4)
was determined as 2.45(4) 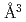 and 3.31(6)
and 3.31(6) 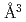 for fcc and hcp phases, respectively, which were significantly larger than the corresponding values for FeD
for fcc and hcp phases, respectively, which were significantly larger than the corresponding values for FeD . The
. The 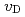 value slightly increased with increasing temperature. This study suggests that only 10% of nickel in iron drastically changes the behaviors of hydrogen in metal. Assuming that
value slightly increased with increasing temperature. This study suggests that only 10% of nickel in iron drastically changes the behaviors of hydrogen in metal. Assuming that 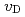 is constant regardless of pressure, the maximum hydrogen content in the Earth's inner core is estimated to be one to two times the amount of hydrogen in the oceans.
is constant regardless of pressure, the maximum hydrogen content in the Earth's inner core is estimated to be one to two times the amount of hydrogen in the oceans.
 Fe
Fe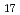 intermetallic compound
intermetallic compoundCao, Y.*; Zhou, H.*; Khmelevskyi, S.*; Lin, K.*; Avdeev, M.*; Wang, C.-W.*; Wang, B.*; Hu, F.*; Kato, Kenichi*; Hattori, Takanori; et al.
Chemistry of Materials, 35(8), p.3249 - 3255, 2023/04
Times Cited Count:1 Percentile:0(Chemistry, Physical)Hydrostatic and chemical pressure are efficient stimuli to alter the crystal structure and are commonly used for tuning electronic and magnetic properties in materials science. However, chemical pressure is difficult to quantify and a clear correspondence between these two types of pressure is still lacking. Here, we study intermetallic candidates for a permanent magnet with a negative thermal expansion (NTE). Based on in situ synchrotron X-ray diffraction, negative chemical pressure is revealed in Ho Fe
Fe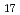 on Al doping and quantitatively evaluated by using temperature and pressure dependence of unit cell volume. A combination of magnetization and neutron diffraction measurements also allowed one to compare the effect of chemical pressure on magnetic ordering with that of hydrostatic pressure. Intriguingly, pressure can be used to control suppression and enhancement of NTE. Electronic structure calculations indicate that pressure affected the top of the majority band with respect to the Fermi level, which has implications for the magnetic stability, which in turn plays a critical role in modulating magnetism and NTE. This work presents a good example of understanding the effect of pressure and utilizing it to control properties of functional materials.
on Al doping and quantitatively evaluated by using temperature and pressure dependence of unit cell volume. A combination of magnetization and neutron diffraction measurements also allowed one to compare the effect of chemical pressure on magnetic ordering with that of hydrostatic pressure. Intriguingly, pressure can be used to control suppression and enhancement of NTE. Electronic structure calculations indicate that pressure affected the top of the majority band with respect to the Fermi level, which has implications for the magnetic stability, which in turn plays a critical role in modulating magnetism and NTE. This work presents a good example of understanding the effect of pressure and utilizing it to control properties of functional materials.
Jiang, X.*; Hattori, Takanori; Xu, X.*; Li, M.*; Yu, C.*; Yu, D.*; Mole, R.*; Yano, Shinichiro*; Chen, J.*; He, L.*; et al.
Materials Horizons, 10(3), p.977 - 982, 2023/03
Times Cited Count:5 Percentile:87.86(Chemistry, Multidisciplinary)As a promising environment-friendly alternative to current vapor-compression refrigeration, solid-state refrigeration based on the barocaloric effect has been attracting world wide attention. Generally, both phases in which a barocaloric effect occurs are present at ambient pressure. Here, instead, we demonstrate that KPF exhibits a colossal barocaloric effect due to the creation of a high-pressure rhombohedral phase. The phase diagram is constructed based on pressure-dependent calorimetric, Raman scattering, and neutron diffraction measurements. The present study is expected to provide an alternative routine to colossal barocaloric effects through the creation of a high-pressure phase.
exhibits a colossal barocaloric effect due to the creation of a high-pressure rhombohedral phase. The phase diagram is constructed based on pressure-dependent calorimetric, Raman scattering, and neutron diffraction measurements. The present study is expected to provide an alternative routine to colossal barocaloric effects through the creation of a high-pressure phase.
Yamaguchi, Toshio*; Yoshida, Koji*; Machida, Shinichi*; Hattori, Takanori
Journal of Molecular Liquids, 365, p.120181_1 - 120181_10, 2022/11
Times Cited Count:1 Percentile:16.81(Chemistry, Physical)Neutron scattering measurements were performed on an aqueous 3 mol/kg NaCl solution in D O at temperature and pressure conditions of 0.1 MPa/298K, 1 GPa/298K, 1 GPa/523K, and 4 GPa/523K. The empirical potential structure refinement method was applied to the obtained data to extract the pair correlation function, coordination number distribution, angular distribution (orientation correlation), and spatial density function (3-D structure). From those results, pressure and temperature dependence of solvation and association of ions and solvent-water structure were discussed.
O at temperature and pressure conditions of 0.1 MPa/298K, 1 GPa/298K, 1 GPa/523K, and 4 GPa/523K. The empirical potential structure refinement method was applied to the obtained data to extract the pair correlation function, coordination number distribution, angular distribution (orientation correlation), and spatial density function (3-D structure). From those results, pressure and temperature dependence of solvation and association of ions and solvent-water structure were discussed.
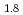 and TiH
and TiH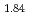 up to 21 GPa by incoherent inelastic neutron scattering
up to 21 GPa by incoherent inelastic neutron scatteringHattori, Takanori; Nakamura, Mitsutaka; Iida, Kazuki*; Machida, Akihiko*; Sano, Asami; Machida, Shinichi*; Arima, Hiroshi*; Oshita, Hidetoshi*; Honda, Takashi*; Ikeda, Kazutaka*; et al.
Physical Review B, 106(13), p.134309_1 - 134309_9, 2022/10
Times Cited Count:0 Percentile:0(Materials Science, Multidisciplinary)Hydrogen vibration excitations of fluorite-type ZrH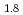 and TiH
and TiH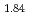 were investigated up to 21 GPa and 4 GPa, respectively, by incoherent inelastic neutron scattering experiments. The first excitation energies increased with pressure, as described by the equations
were investigated up to 21 GPa and 4 GPa, respectively, by incoherent inelastic neutron scattering experiments. The first excitation energies increased with pressure, as described by the equations 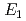 (meV) = 141.4(2) + 1.02(2)
(meV) = 141.4(2) + 1.02(2) (GPa) and
(GPa) and 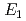 (meV) = 149.4(1) + 1.21(8)
(meV) = 149.4(1) + 1.21(8) (GPa) for ZrH
(GPa) for ZrH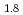 and TiH
and TiH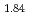 , respectively. Coupling with pressure dependence of lattice parameters, the relations between metal-hydrogen distance (
, respectively. Coupling with pressure dependence of lattice parameters, the relations between metal-hydrogen distance (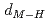 ) and
) and 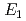 are found to be well described by the equations
are found to be well described by the equations 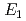 (meV) = 1.62(9)
(meV) = 1.62(9) 10
10
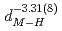 (
(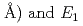 (meV) = 1.47(21)
(meV) = 1.47(21) 10
10
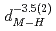 (AA), respectively. The slopes of these curves are much steep compared to the previously reported trend in various fluorite-type metal hydrides at ambient pressure. The hydrogen wave function spreading showed that the local potential field for a hydrogen atom shrinks more intensively than the tetrahedral site. These behavior is likely caused by the rigid metal ion core and the resulting confinement of the hydrogen atom in the narrower potential field at high pressures.
(AA), respectively. The slopes of these curves are much steep compared to the previously reported trend in various fluorite-type metal hydrides at ambient pressure. The hydrogen wave function spreading showed that the local potential field for a hydrogen atom shrinks more intensively than the tetrahedral site. These behavior is likely caused by the rigid metal ion core and the resulting confinement of the hydrogen atom in the narrower potential field at high pressures.
 Fe
Fe O
O solid solutions under high-pressure and high-temperature conditions
solid solutions under high-pressure and high-temperature conditionsYamanaka, Takamitsu*; Hirao, Naohisa*; Nakamoto, Yuki*; Mikouchi, Takashi*; Hattori, Takanori; Komatsu, Kazuki*; Mao, H.-K.*
Physics and Chemistry of Minerals, 49(10), p.41_1 - 41_14, 2022/10
Times Cited Count:0 Percentile:0.02(Materials Science, Multidisciplinary)Magnetic and crystal structure of Mn Fe
Fe O
O solid solutions under high-PT conditions are investigated by neutron diffraction and synchrotron M
solid solutions under high-PT conditions are investigated by neutron diffraction and synchrotron M ssbauer spectroscopy. The ferrimagnetic-paramagnetic transition and tetragonal-cubic transition of Mn
ssbauer spectroscopy. The ferrimagnetic-paramagnetic transition and tetragonal-cubic transition of Mn FeO
FeO spinel occur at 100
spinel occur at 100 C and 180
C and 180 C, respectively, suggesting both the transitions are not coupled. The structure transition temperature decreases with pressure. M
C, respectively, suggesting both the transitions are not coupled. The structure transition temperature decreases with pressure. M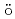 ssbauer experiments and neutron diffraction revealed that the Fe
ssbauer experiments and neutron diffraction revealed that the Fe occupancy in tetrahedral site increases increase with pressure, suggesting Mn
occupancy in tetrahedral site increases increase with pressure, suggesting Mn FeO
FeO phase approaches inverse spinel. Magnetic structure refinement clarified paramagnetic and ferrimagnetic structure of MnFe
phase approaches inverse spinel. Magnetic structure refinement clarified paramagnetic and ferrimagnetic structure of MnFe O
O and Mn
and Mn FeO
FeO . These spinels transform into high-pressure orthorhombic phases at 18.4 and 14.0 GPa, respectively, indicating lower transition pressure with increasing Mn content.
. These spinels transform into high-pressure orthorhombic phases at 18.4 and 14.0 GPa, respectively, indicating lower transition pressure with increasing Mn content.
Yamashita, Keishiro*; Komatsu, Kazuki*; Klotz, S.*; Fabelo, O.*; Fern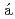 ndez-D
ndez-D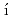 az, M. T.*; Abe, Jun*; Machida, Shinichi*; Hattori, Takanori; Irifune, Tetsuo*; Shimmei, Toru*; et al.
az, M. T.*; Abe, Jun*; Machida, Shinichi*; Hattori, Takanori; Irifune, Tetsuo*; Shimmei, Toru*; et al.
Proceedings of the National Academy of Sciences of the United States of America, 119(40), p.e2208717119_1 - e2208717119_6, 2022/10
Times Cited Count:2 Percentile:22.35(Multidisciplinary Sciences)Here we present the first elucidation of the disordered structure of ice VII, the dominant high-pressure form of water, at 2.2 GPa and 298 K from both single-crystal and powder neutron diffraction techniques. We reveal the three-dimensional atomic distributions from the maximum entropy method and unexpectedly find a ring-like distribution of hydrogen in contrast to the commonly-accepted discrete sites. In addition, total scattering analysis at 274 K clarified the difference in the intermolecular structure from ice VIII, the ordered counterpart of ice VII, despite an identical molecular geometry. Our complementary structure analyses robustly demonstrate the unique disordered structure of ice VII. Furthermore, these noble findings are related to the proton dynamics which drastically vary with pressure, and will contribute to an understanding of the structural origin of anomalous physical properties of ice VII under pressures.
 Fe
Fe O
O spinel and postspinel with elevating pressure
spinel and postspinel with elevating pressureYamanaka, Takamitsu*; Rahman, S.*; Nakamoto, Yuki*; Hattori, Takanori; Jang, B. G.*; Kim, D. Y.*; Mao, H.-K.*
Journal of Physics and Chemistry of Solids, 167, p.110721_1 - 110721_10, 2022/08
Times Cited Count:1 Percentile:15.7(Chemistry, Multidisciplinary)High-pressure neutron diffraction proved that MnFe O
O and Mn
and Mn FeO
FeO spinels transform into CaMn
spinels transform into CaMn O
O -type structure above 18 GPa and 14 GPa, respectively. The transition pressure of Mn
-type structure above 18 GPa and 14 GPa, respectively. The transition pressure of Mn Fe
Fe O
O solutions decreases with increasing Mn content. Synchrotron X-ray M
solutions decreases with increasing Mn content. Synchrotron X-ray M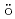 ssbauer experiments revealed that Fe
ssbauer experiments revealed that Fe and Fe
and Fe distribution at the tetrahedral (A) and octahedral (B) sites in the spinel structure changes with pressure. MnFe
distribution at the tetrahedral (A) and octahedral (B) sites in the spinel structure changes with pressure. MnFe O
O and Mn
and Mn FeO
FeO spinels are ferrimagnetic and the CaMn
spinels are ferrimagnetic and the CaMn O
O -type phase is paramagnetic. The temperature dependence of resistivity indicates that both spinels are semiconductors wherein electrons hop between cations at the A and B sites. A pressure-induced shortening of B-B distance promoted conduction via greater electron mobility between adjacent B cations. The Fe
-type phase is paramagnetic. The temperature dependence of resistivity indicates that both spinels are semiconductors wherein electrons hop between cations at the A and B sites. A pressure-induced shortening of B-B distance promoted conduction via greater electron mobility between adjacent B cations. The Fe and Fe
and Fe occupancies at the B sites in MnFe
occupancies at the B sites in MnFe O
O are much larger than those in Mn
are much larger than those in Mn FeO
FeO . The CaMn
. The CaMn O
O -type phase is metallic. Theoretical calculation confirmed the metallic character and Fe d-orbitals strongly renormalized compared to Mn d-orbitals.
-type phase is metallic. Theoretical calculation confirmed the metallic character and Fe d-orbitals strongly renormalized compared to Mn d-orbitals.
Hattori, Takanori
Yukuatsu Gijutsu, 61(7), p.29 - 35, 2022/07
As an example of the application of hydraulic technology, the 6-axis type multi-anvil press "ATSUHIME" in the J-PARC ultra-high pressure neutron diffractometer PLANET and the research on hydrogen in the Earth's core using them are introduced.
Ohashi, Tomonori*; Sakamaki, Tatsuya*; Funakoshi, Kenichi*; Hattori, Takanori; Hisano, Naoki*; Abe, Jun*; Suzuki, Akio*
American Mineralogist, 107(3), p.325 - 335, 2022/03
Times Cited Count:1 Percentile:22.72(Geochemistry & Geophysics)The basaltic glass structure were investigated to 18 GPa using in situ X-ray and neutron diffraction. The O-O coordination number (CN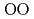 ) starts to rise with maintaining the mean O-O distance (r
) starts to rise with maintaining the mean O-O distance (r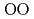 ) above 2-4 GPa, and then CN
) above 2-4 GPa, and then CN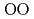 stops increasing and r
stops increasing and r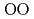 begins to shrink along with the increase in the Al-O coordination number (CN
begins to shrink along with the increase in the Al-O coordination number (CN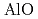 ) above 9 GPa. This is interpreted by the change in the contraction mechanism from tetrahedral network bending to oxygen packing ratio increase via the CN
) above 9 GPa. This is interpreted by the change in the contraction mechanism from tetrahedral network bending to oxygen packing ratio increase via the CN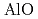 increase. The oxygen packing fraction exceeds the value for dense random packing, suggesting that the oxygen-packing hypothesis cannot account for the pressure-induced structural transformations of silica and silicate glasses. The CN
increase. The oxygen packing fraction exceeds the value for dense random packing, suggesting that the oxygen-packing hypothesis cannot account for the pressure-induced structural transformations of silica and silicate glasses. The CN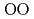 increase at 2-4 GPa reflects the elastic softening of silicate glass, which may causes anomalous elastic moduli of basaltic glass at
increase at 2-4 GPa reflects the elastic softening of silicate glass, which may causes anomalous elastic moduli of basaltic glass at  2 GPa.
2 GPa.
Zhang, W. Q.*; Yamaguchi, Toshio*; Fang, C. H.*; Yoshida, Koji*; Zhou, Y. Q.*; Zhu, F. Y.*; Machida, Shinichi*; Hattori, Takanori; Li, W.*
Journal of Molecular Liquids, 348, p.118080_1 - 118080_11, 2022/02
Times Cited Count:2 Percentile:34.79(Chemistry, Physical)The ion hydration and association and hydrogen-bonded water structure in an aqueous 3 mol/kg RbCl solution were investigated at 298 K/0.1 MPa, 298 K/1 GPa, 523 K/1 GPa, and 523 K/4 GPa by neutron diffraction combined with EPSR methods. The second hydration layer of Rb and Cl
and Cl becomes evident under elevated pressure and temperature conditions. The average oxygen coordination number of Rb
becomes evident under elevated pressure and temperature conditions. The average oxygen coordination number of Rb (Cl
(Cl ) in the first hydration layer increases from 6.3 (5.9) ambient pressure to 8.9 (9.1) at 4 GPa, while decreasing coordination distance from 0.290 nm (0.322 nm) to 0.288 nm (0.314 nm). The orientation of the water dipole in the first solvation shell of Rb
) in the first hydration layer increases from 6.3 (5.9) ambient pressure to 8.9 (9.1) at 4 GPa, while decreasing coordination distance from 0.290 nm (0.322 nm) to 0.288 nm (0.314 nm). The orientation of the water dipole in the first solvation shell of Rb and a central water molecule is sensitive to pressure, but that in the first solvation shell of Cl
and a central water molecule is sensitive to pressure, but that in the first solvation shell of Cl does not change very much. The number of contact-ion pairs Rb
does not change very much. The number of contact-ion pairs Rb -Cl
-Cl decreases with elevated temperature and increases with elevated pressure. Water molecules are closely packed, and the tetrahedral hydrogen-bonded network of water molecules no longer exists in extreme conditions.
decreases with elevated temperature and increases with elevated pressure. Water molecules are closely packed, and the tetrahedral hydrogen-bonded network of water molecules no longer exists in extreme conditions.
Hattori, Takanori; Kawamura, Seiko; Kawasaki, Takuro
High Pressure Research, 42(2), p.226 - 235, 2022/00
Times Cited Count:0 Percentile:0(Physics, Multidisciplinary)We have developed a hybrid piston cylinder cell for quasi-elastic neutron scattering (QENS) experiments up to about 1 GPa. It consists of a fretted cylinder made of the high tensile steel (SNCM439) liner and the Al alloy (NA700) jacket. Performance tests revealed that the cell can withstand a pressure of 0.8 GPa without irreversible damage and has 4.4 times larger neutron transmission at 3.14 meV (5.10 in wavelength) than that of a conventional CuBe monobloc cylinder. Combined with the sample assembly devised for suppressing multiple scattering, high quality QENS spectra of water were obtained up to 0.8 GPa. This study illustrates the efficacy of the hybrid cylinders not only for increasing maximum available pressure but also manipulating the available pressure and the signal intensity, depending on the purpose of the experiments.
in wavelength) than that of a conventional CuBe monobloc cylinder. Combined with the sample assembly devised for suppressing multiple scattering, high quality QENS spectra of water were obtained up to 0.8 GPa. This study illustrates the efficacy of the hybrid cylinders not only for increasing maximum available pressure but also manipulating the available pressure and the signal intensity, depending on the purpose of the experiments.
Wang, X.*; Tang, X.*; Zhang, P.*; Wang, Y.*; Gao, D.*; Liu, J.*; Hui, K.*; Wang, Y.*; Dong, X.*; Hattori, Takanori; et al.
Journal of Physical Chemistry Letters (Internet), 12(50), p.12055 - 12061, 2021/12
Times Cited Count:6 Percentile:44.89(Chemistry, Physical)Substituted polyacetylene is expected to improve the chemical stability, physical properties, and additional functions of the polyacetylene backbones, but its diversity is very limited. Here, by applying external pressure on solid acetylenedicarboxylic acid, we report the first crystalline poly-dicarboxylacetylene with every carbon on the trans-polyacetylene backbone bonded to a carboxyl group, which is very hard to synthesize by traditional methods. This unique structure combines the extremely high content of carbonyl groups and high conductivity of a polyacetylene backbone, which exhibits a high specific capacity and excellent cycling/rate performance as a Li-ion battery (LIB) anode. We present a completely functionalized crystalline polyacetylene and provide a high-pressure solution for the synthesis of polymeric LIB materials and other polymeric materials with a high content of active groups.