Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cell
-hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsubata, Yasuhiro
Solvent Extraction Research and Development, Japan, 31(1), p.1 - 11, 2024/00
A demonstration test was performed to separate minor actinides (MA; Am and Cm) by  -hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm
-hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm in
in  -dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were
-dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were  99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
 -hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cell
-hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsutsui, Nao; Tsubata, Yasuhiro; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 37(7), p.489 - 499, 2019/11
Times Cited Count:15 Percentile:52.81(Chemistry, Multidisciplinary)A continuous counter-current experiment to separate minor actinides (MAs: Am and Cm) was performed with  -hexaochyl nitrilotriacetamide (HONTA) as an extractant. Nitric acid of 0.08 M (mol/dm
-hexaochyl nitrilotriacetamide (HONTA) as an extractant. Nitric acid of 0.08 M (mol/dm ) containing MAs and rare earths (REs) recovered from high-level waste was used as the Feed, and the experiment was conducted for 14 h. The ratios of Am and Cm recovered into the MA fraction measured 94.9% and 78.9%, respectively. HONTA hardly extracted Y, La, and Eu in the Feed (99.9% for Y, 99.9% for La, and 96.7% for Eu), most of which were distributed to the RE fraction. A portion of Nd was extracted by HONTA, and consequently the ratio of Nd in the RE fraction was 83.5%. The concentrations of MAs and some REs in each stage were calculated using a simulation code, and the results are consistent with the experimental values. This code indicates that the ratios of MAs in the MA fraction and REs in the RE fraction could be
) containing MAs and rare earths (REs) recovered from high-level waste was used as the Feed, and the experiment was conducted for 14 h. The ratios of Am and Cm recovered into the MA fraction measured 94.9% and 78.9%, respectively. HONTA hardly extracted Y, La, and Eu in the Feed (99.9% for Y, 99.9% for La, and 96.7% for Eu), most of which were distributed to the RE fraction. A portion of Nd was extracted by HONTA, and consequently the ratio of Nd in the RE fraction was 83.5%. The concentrations of MAs and some REs in each stage were calculated using a simulation code, and the results are consistent with the experimental values. This code indicates that the ratios of MAs in the MA fraction and REs in the RE fraction could be  99% by optimizing separation conditions.
99% by optimizing separation conditions.
 -tetradodecyldiglycolamide (TDdDGA) by using mixer-settler extractors in a hot cell
-tetradodecyldiglycolamide (TDdDGA) by using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya; Hotoku, Shinobu; Kawasaki, Tomohiro*; Sagawa, Hiroshi*; Tsutsui, Nao; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 37(1), p.27 - 37, 2019/00
Times Cited Count:21 Percentile:65.06(Chemistry, Multidisciplinary)A continuous counter-current experiment using TDdDGA was performed using mixer-settler extractors installed in a hot cell. Nitric acid containing minor actinides (MAs: Am and Cm), rare earths (REs: Y, La, Nd, and Eu), and other fission products (Sr, Cs, Zr, Mo, Ru, Rh, and Pd) was fed to the extractor. TDdDGA effectively extracted MAs and REs from the feed, while other fission products were barely extracted. The extracted MAs and REs were back-extracted by bringing them in contact with 0.02 mol/dm nitric acid, and they were collected as the MA-RE fraction. The proportions of MA and RE in the MA-RE fraction were
nitric acid, and they were collected as the MA-RE fraction. The proportions of MA and RE in the MA-RE fraction were  98% and
98% and  86%, respectively. These results demonstrated the applicability of TDdDGA as an extractant for MAs and REs.
86%, respectively. These results demonstrated the applicability of TDdDGA as an extractant for MAs and REs.
 -dialkylamides using multistage mixer-settler extractors
-dialkylamides using multistage mixer-settler extractorsBan, Yasutoshi; Hotoku, Shinobu; Tsutsui, Nao; Suzuki, Asuka; Tsubata, Yasuhiro; Matsumura, Tatsuro
Procedia Chemistry, 21, p.156 - 161, 2016/12
Times Cited Count:5 Percentile:94.32(Chemistry, Inorganic & Nuclear)A continuous counter-current experiment was carried out to demonstrate the validity of a process using  -dialkylamides for recovering U and Pu. This process consisted of two cycles, and the 1st cycle and the 2nd cycle employed
-dialkylamides for recovering U and Pu. This process consisted of two cycles, and the 1st cycle and the 2nd cycle employed  -di(2-ethylhexyl)-2,2-dimethylpropanamide and
-di(2-ethylhexyl)-2,2-dimethylpropanamide and  -di(2-ethylhexyl)butanamide as extractants, respectively. The feed solution for the 1st cycle was 5.1 mol/dm
-di(2-ethylhexyl)butanamide as extractants, respectively. The feed solution for the 1st cycle was 5.1 mol/dm (M) nitric acid containing 0.92 M U, 1.6 mM Pu, and 0.6 mM Np. The raffinate collected in the 1st cycle was used as the feed for the 2nd cycle. The ratios of U recovered in the U fraction and U-Pu fraction were 99.1% and 0.8%, respectively. The ratio of Pu recovered in the U-Pu fraction was 99.7%. The concentration ratio of U with respect to Pu in the U-Pu fraction was 9, and this indicated that Pu was not isolated. The decontamination factor of U with respect to Pu in the U fraction was obtained as 4.5
(M) nitric acid containing 0.92 M U, 1.6 mM Pu, and 0.6 mM Np. The raffinate collected in the 1st cycle was used as the feed for the 2nd cycle. The ratios of U recovered in the U fraction and U-Pu fraction were 99.1% and 0.8%, respectively. The ratio of Pu recovered in the U-Pu fraction was 99.7%. The concentration ratio of U with respect to Pu in the U-Pu fraction was 9, and this indicated that Pu was not isolated. The decontamination factor of U with respect to Pu in the U fraction was obtained as 4.5 10
10 . These results supported the validity of the proposed process.
. These results supported the validity of the proposed process.
Ban, Yasutoshi; Hotoku, Shinobu; Tsutsui, Nao; Suzuki, Asuka; Matsumura, Tatsuro
Proceedings of 5th International Conference on Asian Nuclear Prospects 2016 (ANUP 2016) (CD-ROM), 2 Pages, 2016/10
A continuous counter-current experiment was conducted using mixer-settler extractors installed in a hot cell.  -Di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) was used as the extractant for U. The experimental results showed that DEHDMPA can effectively extract U from nitric acid, and the recovery of U in the U fraction was obtained as 99.6%.
-Di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) was used as the extractant for U. The experimental results showed that DEHDMPA can effectively extract U from nitric acid, and the recovery of U in the U fraction was obtained as 99.6%.
Suzuki, Asuka; Hotoku, Shinobu; Ban, Yasutoshi; Tsutsui, Nao; Tsubata, Yasuhiro; Matsumura, Tatsuro
Proceedings of 5th International Conference on Asian Nuclear Prospects 2016 (ANUP 2016) (CD-ROM), 2 Pages, 2016/10
The Japan Atomic Energy Agency (JAEA) conducts extensive research and development (R&D) activities toward the development of aqueous separation processes for spent nuclear fuel at the Nuclear Fuel Cycle Safety Engineering Research Facility-Back-end Fuel Cycle Key Element Research Facility (NUCEF-BECKY). BECKY is equipped with three hot cells, several glove boxes, and fume hoods. One of the hot cells, called the process cell, contains bench scale spent fuel test equipment used for U, Pu, and other spent fuel material. In this study, we describe an equipment used for aqueous separation processes in the process cell.
 -dialkylamides (DEHDMPA and DEHBA) in mixer-settler extractors
-dialkylamides (DEHDMPA and DEHBA) in mixer-settler extractorsBan, Yasutoshi; Hotoku, Shinobu; Tsutsui, Nao; Tsubata, Yasuhiro; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 34(1), p.37 - 47, 2016/01
Times Cited Count:9 Percentile:29.47(Chemistry, Multidisciplinary)The extraction properties of  -di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) and
-di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) and  -di(2-ethylhexyl)butanamide (DEHBA) for Np(V) and Np(VI) were studied by a batch method using various nitrate ion concentrations. The distribution ratios of Np(VI) obtained with DEHDMPA and DEHBA exceeded unity when the nitrate ion concentration was
-di(2-ethylhexyl)butanamide (DEHBA) for Np(V) and Np(VI) were studied by a batch method using various nitrate ion concentrations. The distribution ratios of Np(VI) obtained with DEHDMPA and DEHBA exceeded unity when the nitrate ion concentration was  3 mol/L. DEHDMPA and DEHBA barely extracted Np(V), and the maximum distribution ratios were 0.4 and 0.2 when DEHDMPA and DEHBA were used as extractants, respectively. A continuous counter-current experiment was performed to evaluate the behavior of Np in a process comprising two cycles. The ratio of Np recovered to the U fraction and U-Pu fraction were 63.7% and 29.1%, respectively. The behavior of Np suggested that the valence state of Np changed from Np(V) to Np(IV) or Np(VI) after the 1st experimental cycle. The recoveries of U and Pu to the U fraction stream and the U-Pu fraction stream were 99.9% and 99.8%, respectively.
3 mol/L. DEHDMPA and DEHBA barely extracted Np(V), and the maximum distribution ratios were 0.4 and 0.2 when DEHDMPA and DEHBA were used as extractants, respectively. A continuous counter-current experiment was performed to evaluate the behavior of Np in a process comprising two cycles. The ratio of Np recovered to the U fraction and U-Pu fraction were 63.7% and 29.1%, respectively. The behavior of Np suggested that the valence state of Np changed from Np(V) to Np(IV) or Np(VI) after the 1st experimental cycle. The recoveries of U and Pu to the U fraction stream and the U-Pu fraction stream were 99.9% and 99.8%, respectively.
 in a genuine dissolver solution from the results of extraction simulation by PARC-L code
in a genuine dissolver solution from the results of extraction simulation by PARC-L codeAsakura, Toshihide; Hotoku, Shinobu; Morita, Yasuji
Journal of Nuclear Science and Technology, 52(12), p.1552 - 1561, 2015/12
Times Cited Count:1 Percentile:9.74(Nuclear Science & Technology)With a genuine spent fuel soltuion (a dissolver solution), a laboratory-scale reprocessing experiment of an extraction-separation process was performed using mixer-settlers as extactors. In the experiment, n-butyraldehyde was utilized as a reducing reagent of Np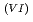 O
O
 to Np
to Np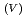 O
O
 for the purpose to distinguish Np
for the purpose to distinguish Np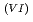 O
O
 from Np
from Np . From the Np concentration in the aqueous phase, Np would be extracted from the dissolver solution together with U and Pu. The scrutiny of Np behavior was performed utilizing 66 cases of calculation results by a Japan Atomic Energy Agency open extraction simulation code, the Programm for Advanced Extraction with Radiation Effect Calculation-Lightened version. From the scrutiny, the authors found that the calculation result with 60% of Np
. From the Np concentration in the aqueous phase, Np would be extracted from the dissolver solution together with U and Pu. The scrutiny of Np behavior was performed utilizing 66 cases of calculation results by a Japan Atomic Energy Agency open extraction simulation code, the Programm for Advanced Extraction with Radiation Effect Calculation-Lightened version. From the scrutiny, the authors found that the calculation result with 60% of Np in the dissolver solution represented the best experimental extraction-separtion behavior of Np. Therefore, it was supposed that the dissolver solution contained sufficient proportion of Np
in the dissolver solution represented the best experimental extraction-separtion behavior of Np. Therefore, it was supposed that the dissolver solution contained sufficient proportion of Np to affect the extraction-separation behavior of Np.
to affect the extraction-separation behavior of Np.
 -dialkylamides as extractants for U and Pu by continuous counter-current extractors
-dialkylamides as extractants for U and Pu by continuous counter-current extractorsBan, Yasutoshi; Hotoku, Shinobu; Tsubata, Yasuhiro; Tsutsui, Nao; Matsumura, Tatsuro
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.1147 - 1152, 2015/09
Extraction properties of  -di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA),
-di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA),  -di(2-ethylhexyl)butanamide (DEHBA), and some of their degradation products for the metal elements Zr, Mo, Ru, Rh, and Pd were studied using a single-stage batch method, and the results showed that the degradation products barely extracted these metal elements. Furthermore, separation performance of DEHDMPA and DEHBA for U and Pu in a continuous counter-current process was evaluated using a calculation code, and it was confirmed that the calculated values of U concentration in the U fraction and U and Pu concentrations in the U-Pu fraction were similar to those measured experimentally. These results supported the applicability of DEHDMPA and DEHBA as extractants for separation processes and the validity of the calculation code for estimating the separation performance of the process.
-di(2-ethylhexyl)butanamide (DEHBA), and some of their degradation products for the metal elements Zr, Mo, Ru, Rh, and Pd were studied using a single-stage batch method, and the results showed that the degradation products barely extracted these metal elements. Furthermore, separation performance of DEHDMPA and DEHBA for U and Pu in a continuous counter-current process was evaluated using a calculation code, and it was confirmed that the calculated values of U concentration in the U fraction and U and Pu concentrations in the U-Pu fraction were similar to those measured experimentally. These results supported the applicability of DEHDMPA and DEHBA as extractants for separation processes and the validity of the calculation code for estimating the separation performance of the process.
 -di(2-
-di(2-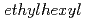 )-2,2-dimethylpropanamide (DEHDMPA) and
)-2,2-dimethylpropanamide (DEHDMPA) and  -di(2-
-di(2-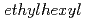 )butanamide (DEHBA) using mixer-settlers in the presence of degradation products of DEHDMPA and DEHBA
)butanamide (DEHBA) using mixer-settlers in the presence of degradation products of DEHDMPA and DEHBABan, Yasutoshi; Hotoku, Shinobu; Tsubata, Yasuhiro; Tsutsui, Nao; Matsumura, Tatsuro
Solvent Extraction Research and Development, Japan, 22(1), p.47 - 55, 2015/00
Two sets of continuous counter-current experiments using mixer-setters were performed for evaluating extraction properties of  -dialkylamides toward U and Pu in the presence of their degradation products. The 1st cycle employed
-dialkylamides toward U and Pu in the presence of their degradation products. The 1st cycle employed  -di(2-
-di(2-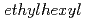 )-2,2-dimethylpropanamide (DEHDMPA) for selective extraction of U(VI), and the 2nd cycle employed
)-2,2-dimethylpropanamide (DEHDMPA) for selective extraction of U(VI), and the 2nd cycle employed  -di(2-
-di(2-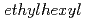 )butanamide (DEHDBA) for co-extraction of U(VI) and Pu(IV). Degradation products were added to the organic phase of each cycle. Most of U was effectively extracted by DEHDMPA, and the ratio of U recovered to the U fraction was 99.57%. DEHDMPA barely extracted Pu, and the decontamination factor of U with respect to Pu in the U fraction was 1.1
)butanamide (DEHDBA) for co-extraction of U(VI) and Pu(IV). Degradation products were added to the organic phase of each cycle. Most of U was effectively extracted by DEHDMPA, and the ratio of U recovered to the U fraction was 99.57%. DEHDMPA barely extracted Pu, and the decontamination factor of U with respect to Pu in the U fraction was 1.1  10
10 . The raffinate of the 1st cycle was used as the feed in the 2nd cycle, and the residual U and almost all Pu were effectively extracted by DEHBA. The degradation products had no detrimental effects on the two-phase separation and the operation of mixer-settlers.
. The raffinate of the 1st cycle was used as the feed in the 2nd cycle, and the residual U and almost all Pu were effectively extracted by DEHBA. The degradation products had no detrimental effects on the two-phase separation and the operation of mixer-settlers.
 -di(2-ethylhexyl)-2,2-dimetnylpropanamide (DEHDMPA) and
-di(2-ethylhexyl)-2,2-dimetnylpropanamide (DEHDMPA) and  -di(2-ethylhexyl)butanamide (DEHBA) using mixer-settler extractors
-di(2-ethylhexyl)butanamide (DEHBA) using mixer-settler extractorsBan, Yasutoshi; Hotoku, Shinobu; Tsubata, Yasuhiro; Morita, Yasuji
Solvent Extraction and Ion Exchange, 32(4), p.348 - 364, 2014/05
Times Cited Count:9 Percentile:31.46(Chemistry, Multidisciplinary)Extraction properties of  -di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) for nitric acid, U(VI), and Pu(IV) were studied, and the distribution ratio equations were derived for each chemical species. A continuous counter-current experiment was performed using mixer-settler extractors with two types of monoamides, DEHDMPA and
-di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) for nitric acid, U(VI), and Pu(IV) were studied, and the distribution ratio equations were derived for each chemical species. A continuous counter-current experiment was performed using mixer-settler extractors with two types of monoamides, DEHDMPA and  -di(2-ethylhexyl)butanamide (DEHBA), as extractants. DEHDMPA exclusively extracted U from the feed, and the ratio of U recovered in the U fraction stream was 99.93%. Almost all Pu were extracted by DEHBA, and the recovery of Pu in the U-Pu fraction stream was 99.94%. Concentrations of U and Pu in mixer-settlers were calculated using a simulation code, which confirmed that the calculation was effective for estimating the U concentration in the U fraction stream, and the U and Pu concentrations in the U-Pu fraction stream.
-di(2-ethylhexyl)butanamide (DEHBA), as extractants. DEHDMPA exclusively extracted U from the feed, and the ratio of U recovered in the U fraction stream was 99.93%. Almost all Pu were extracted by DEHBA, and the recovery of Pu in the U-Pu fraction stream was 99.94%. Concentrations of U and Pu in mixer-settlers were calculated using a simulation code, which confirmed that the calculation was effective for estimating the U concentration in the U fraction stream, and the U and Pu concentrations in the U-Pu fraction stream.
Inagawa, Jun; Hotoku, Shinobu; Oda, Tetsuzo; Aoyagi, Noboru; Magara, Masaaki
JAEA-Technology 2014-007, 48 Pages, 2014/03
In safeguard laboratory (SGL) facility of Nuclear Science Research Institute of JAEA, uranium hexafluoride (UF ) of enriched uranium of various enrichment was used for research and development of a spectrometric method for the determination of the enrichment of uranium in April 1983 through March 1993. After completion of this R&D, the UF
) of enriched uranium of various enrichment was used for research and development of a spectrometric method for the determination of the enrichment of uranium in April 1983 through March 1993. After completion of this R&D, the UF has been stored in SGL facility. It was decided that the UF
has been stored in SGL facility. It was decided that the UF is carried to out of the facility, because the SGL facility will be decommissioning until March 2015. To transport and store in safety after transportation, it is necessary that the UF
is carried to out of the facility, because the SGL facility will be decommissioning until March 2015. To transport and store in safety after transportation, it is necessary that the UF should be converted to stable chemical form. Hydrolysis of UF
should be converted to stable chemical form. Hydrolysis of UF to uranyl fluoride (UO
to uranyl fluoride (UO F
F ) and evaporation to solid state were selected for the stabilization method. The equipment for hydrolysis and evaporation was installed in the SGL facility. Stabilization was operated in this equipment, and all of the UF
) and evaporation to solid state were selected for the stabilization method. The equipment for hydrolysis and evaporation was installed in the SGL facility. Stabilization was operated in this equipment, and all of the UF in the SGL facility was converted to UO
in the SGL facility was converted to UO F
F solid state in October 2012 through August 2013. In this report, results of examination and operation for stabilization of UF
solid state in October 2012 through August 2013. In this report, results of examination and operation for stabilization of UF were reported.
were reported.
 -di(2-ethylhexyl)butanamide (DEHBA) in mixer-settler extractors
-di(2-ethylhexyl)butanamide (DEHBA) in mixer-settler extractorsBan, Yasutoshi; Hotoku, Shinobu; Tsubata, Yasuhiro; Morita, Yasuji
Solvent Extraction and Ion Exchange, 31(6), p.590 - 603, 2013/09
Times Cited Count:10 Percentile:37.86(Chemistry, Multidisciplinary)The recovery of U and Pu from nitric acid using the monoamide extractant  -di(2-ethylhexyl)butanamide (DEHBA) in mixer-settler extractors was calculated using a simulation code, and a continuous counter-current experiment using mixer-settler extractors was performed. The flow rate, stage number, and nitric acid concentration were chosen as the parameters for the calculation, and the appropriate experimental conditions for separating U from Pu were determined. The results of the continuous counter-current experiment showed that the percentages of U and Pu extracted using 1.5 mol/dm
-di(2-ethylhexyl)butanamide (DEHBA) in mixer-settler extractors was calculated using a simulation code, and a continuous counter-current experiment using mixer-settler extractors was performed. The flow rate, stage number, and nitric acid concentration were chosen as the parameters for the calculation, and the appropriate experimental conditions for separating U from Pu were determined. The results of the continuous counter-current experiment showed that the percentages of U and Pu extracted using 1.5 mol/dm (M) DEHBA from 4 M nitric acid were
(M) DEHBA from 4 M nitric acid were  99.9% and 97.84%, respectively.
99.9% and 97.84%, respectively.
 Sn content in spent nuclear fuel sample
Sn content in spent nuclear fuel sampleAsai, Shiho; Toshimitsu, Masaaki; Hanzawa, Yukiko; Suzuki, Hideya; Shinohara, Nobuo; Inagawa, Jun; Okumura, Keisuke; Hotoku, Shinobu; Kimura, Takaumi; Suzuki, Kensuke*; et al.
Journal of Nuclear Science and Technology, 50(6), p.556 - 562, 2013/06
Times Cited Count:11 Percentile:64.2(Nuclear Science & Technology)The  Sn content in a spent nuclear fuel solution was determined by ICP-MS for its inventory estimation in high-level radioactive waste. An irradiated UO
Sn content in a spent nuclear fuel solution was determined by ICP-MS for its inventory estimation in high-level radioactive waste. An irradiated UO fuel was used as a sample to evaluate the reliability of the methodology. Prior to the measurement, Sn was separated from
fuel was used as a sample to evaluate the reliability of the methodology. Prior to the measurement, Sn was separated from  Te, which causes major isobaric interference in the determination of
Te, which causes major isobaric interference in the determination of  Sn content, along with highly radioactive coexisting elements using an anion-exchange column. The absence of counts attributed to Te in the Sn-containing effluent indicates that Te was completely removed. After washing, Sn retained on the column was readily eluted with 1 M HNO
Sn content, along with highly radioactive coexisting elements using an anion-exchange column. The absence of counts attributed to Te in the Sn-containing effluent indicates that Te was completely removed. After washing, Sn retained on the column was readily eluted with 1 M HNO . The isotope ratios of Sn were successfully determined and showed good agreement with those obtained through ORIGEN2 calculations. The results reported in this paper are the first experimental values of
. The isotope ratios of Sn were successfully determined and showed good agreement with those obtained through ORIGEN2 calculations. The results reported in this paper are the first experimental values of  Sn content in the spent nuclear fuel solution originating in spent nuclear fuel irradiated at a nuclear power plant in Japan.
Sn content in the spent nuclear fuel solution originating in spent nuclear fuel irradiated at a nuclear power plant in Japan.
 -di(2-ethylhexyl)butanamide for mutual separation of U(VI) and Pu(IV) by continuous counter-current extraction with mixer-settler extractors
-di(2-ethylhexyl)butanamide for mutual separation of U(VI) and Pu(IV) by continuous counter-current extraction with mixer-settler extractorsBan, Yasutoshi; Hotoku, Shinobu; Morita, Yasuji
Journal of Nuclear Science and Technology, 49(6), p.588 - 594, 2012/06
Times Cited Count:11 Percentile:63.41(Nuclear Science & Technology)Continuous counter-current extraction using  -di(2-ethylhexyl)- butanamide (DEHBA) as an extractant was performed with mixer-settler type extractors consisting of U-Pu extraction, scrub, U recovery, Pu back-extraction, and U back-extraction steps. The feed solution used in the continuous counter-current extraction was 3 mol/dm
-di(2-ethylhexyl)- butanamide (DEHBA) as an extractant was performed with mixer-settler type extractors consisting of U-Pu extraction, scrub, U recovery, Pu back-extraction, and U back-extraction steps. The feed solution used in the continuous counter-current extraction was 3 mol/dm (M) nitric acid containing U(VI), Pu(IV), and simulated fission products. More than 99.9% of U(VI) and Pu(IV) in the feed was extracted by 1.9 M DEHBA at the U-Pu extraction step. The extracted Pu(IV) was back-extracted via contact with 0.3 M nitric acid in the Pu back-extraction step, and the ratio of Pu(IV) distributed to the Pu fraction stream was
(M) nitric acid containing U(VI), Pu(IV), and simulated fission products. More than 99.9% of U(VI) and Pu(IV) in the feed was extracted by 1.9 M DEHBA at the U-Pu extraction step. The extracted Pu(IV) was back-extracted via contact with 0.3 M nitric acid in the Pu back-extraction step, and the ratio of Pu(IV) distributed to the Pu fraction stream was  82%. It was confirmed that 1.9 M DEHBA effectively recovered U(VI) in the U recovery step, and the ratio of U(VI) in the Pu fraction stream was less than 1%. The extracted U(VI) was back-extracted in the U back-extraction step, and more than 98% of U(VI) was recovered in the U fraction stream.
82%. It was confirmed that 1.9 M DEHBA effectively recovered U(VI) in the U recovery step, and the ratio of U(VI) in the Pu fraction stream was less than 1%. The extracted U(VI) was back-extracted in the U back-extraction step, and more than 98% of U(VI) was recovered in the U fraction stream.
 -di(2-ethylhexyl)butanamide in continuous counter-current extraction with mixer-settler extractor
-di(2-ethylhexyl)butanamide in continuous counter-current extraction with mixer-settler extractorBan, Yasutoshi; Hotoku, Shinobu; Morita, Yasuji
Solvent Extraction and Ion Exchange, 30(2), p.142 - 155, 2012/02
Times Cited Count:7 Percentile:29.12(Chemistry, Multidisciplinary)The extraction properties of 1.5 mol/dm (M)
(M)  -di-(2-ethyl-hexyl)butanamide (DEHBA) diluted with
-di-(2-ethyl-hexyl)butanamide (DEHBA) diluted with  -dodecane toward U(VI) and Pu(IV) were studied using a single stage batch method, and the distribution ratios toward U(VI) and Pu(IV) were, respectively, obtained as follows:
-dodecane toward U(VI) and Pu(IV) were studied using a single stage batch method, and the distribution ratios toward U(VI) and Pu(IV) were, respectively, obtained as follows: 
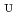 = 1.4[NO
= 1.4[NO
 ]
]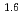 [DEHBA
[DEHBA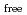 ]
] and
and 
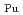 = 0.11[NO
= 0.11[NO
 ]
]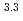 [DEHBA
[DEHBA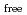 ]
] . A continuous counter-current experiment with 1.5 M DEHBA as an extractant was performed using mixer-settler extractors. The ratios of U(VI) and Pu(IV) extracted by 1.5 M DEHBA in the U-Pu extraction step were more than 99.9%. The extracted Pu(IV) was back-extracted using 0.67 M nitric acid, and more than 97% of Pu(IV) in the feed was recovered in the Pu fraction. The present results indicated that DEHBA works as an extractant for mutual separation of U(VI) and Pu(IV) by adjusting the nitric acid concentration without using Pu(IV) reductants.
. A continuous counter-current experiment with 1.5 M DEHBA as an extractant was performed using mixer-settler extractors. The ratios of U(VI) and Pu(IV) extracted by 1.5 M DEHBA in the U-Pu extraction step were more than 99.9%. The extracted Pu(IV) was back-extracted using 0.67 M nitric acid, and more than 97% of Pu(IV) in the feed was recovered in the Pu fraction. The present results indicated that DEHBA works as an extractant for mutual separation of U(VI) and Pu(IV) by adjusting the nitric acid concentration without using Pu(IV) reductants.
 ,
, -di(2-ethylhexyl)-2,2-dimethylpropanamide
-di(2-ethylhexyl)-2,2-dimethylpropanamideBan, Yasutoshi; Hotoku, Shinobu; Morita, Yasuji
Solvent Extraction and Ion Exchange, 29(4), p.519 - 533, 2011/07
Times Cited Count:14 Percentile:45.07(Chemistry, Multidisciplinary)A continuous counter-current extraction experiment for selective extraction of U(VI) using  ,
, -di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) as an extractant was carried out with mixer-settler type extractors consisting of a U extraction step, a scrub step, and a U back-extraction step. DEHDMPA selectively extracted U(VI) over Pu(IV), and the decontamination factor against Pu(IV) in the U fraction was 990. Fractional distributions of U(VI) in the U fraction and Pu(IV) in the raffinate were 94.5% and 99.9%, respectively. Numerical simulation for calculating U(VI) and Pu(IV) concentrations in each stage of the mixer-settlers was performed. The calculated values agreed with experimentally measured U(VI) and Pu(IV) concentrations in the U extraction step, and also agreed with experimentally measured U(VI) concentrations in the scrub step.
-di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) as an extractant was carried out with mixer-settler type extractors consisting of a U extraction step, a scrub step, and a U back-extraction step. DEHDMPA selectively extracted U(VI) over Pu(IV), and the decontamination factor against Pu(IV) in the U fraction was 990. Fractional distributions of U(VI) in the U fraction and Pu(IV) in the raffinate were 94.5% and 99.9%, respectively. Numerical simulation for calculating U(VI) and Pu(IV) concentrations in each stage of the mixer-settlers was performed. The calculated values agreed with experimentally measured U(VI) and Pu(IV) concentrations in the U extraction step, and also agreed with experimentally measured U(VI) concentrations in the scrub step.
 Se and
Se and  Cs in spent nuclear fuel for inventory estimation of high-level radioactive wastes
Cs in spent nuclear fuel for inventory estimation of high-level radioactive wastesAsai, Shiho; Hanzawa, Yukiko; Okumura, Keisuke; Shinohara, Nobuo; Inagawa, Jun; Hotoku, Shinobu; Suzuki, Kensuke*; Kaneko, Satoru*
Journal of Nuclear Science and Technology, 48(5), p.851 - 854, 2011/05
Times Cited Count:25 Percentile:86.49(Nuclear Science & Technology)Hotoku, Shinobu; Morita, Yasuji
JAEA-Technology 2009-052, 16 Pages, 2009/10
The UF is one of the most important U chemical forms in nuclear fuel cycle, which is used in the U enrichment process and in the study of fluoride volatility process, one of the dry reprocessing methods. Normally, UF
is one of the most important U chemical forms in nuclear fuel cycle, which is used in the U enrichment process and in the study of fluoride volatility process, one of the dry reprocessing methods. Normally, UF is confined in the solid state in the cylinder container and handled as gas by adjusting the temperature and pressure. Since it is highly reactive with water vapor in the air, it must be carefully handled. By the reaction with water vapor, particle of UO
is confined in the solid state in the cylinder container and handled as gas by adjusting the temperature and pressure. Since it is highly reactive with water vapor in the air, it must be carefully handled. By the reaction with water vapor, particle of UO F
F appeared as a white cloud and corrosive HF gas are released to the atmosphere. The purpose of this report is to describe safety handling for clean out of empty UF
appeared as a white cloud and corrosive HF gas are released to the atmosphere. The purpose of this report is to describe safety handling for clean out of empty UF cylinder and to summarize physical and chemical properties of uranium compounds in relation to treatment for UF
cylinder and to summarize physical and chemical properties of uranium compounds in relation to treatment for UF . The clean-out of the UF
. The clean-out of the UF cylinder was carried out successfully by trapping the generated UO
cylinder was carried out successfully by trapping the generated UO F
F and HF adequately in a temporary globe box made of the PVC that set up in a laboratory hood.
and HF adequately in a temporary globe box made of the PVC that set up in a laboratory hood.
Asakura, Toshihide; Hotoku, Shinobu; Ban, Yasutoshi; Matsumura, Masakazu; Morita, Yasuji
Journal of Nuclear and Radiochemical Sciences, 6(3), p.271 - 274, 2005/12
Tc extraction and separation experiments were performed basing on PUREX technique with using spent UO fuel with burn-up of 44 GWd/t. The experimental results were examined with performing calculations by a simulation code ESSCAR (Extraction System Simulation Code for Advanced Reprocessing). It was demonstrated that Tc can be almost quantitatively extracted from a dissolver solution and that Tc can also be almost quantitatively recovered by scrubbing. Further, it was clearly presented from the calculation results of ESSCAR that the extraction mechanism of Tc is dominated by the synergistic effect of Zr and U.
fuel with burn-up of 44 GWd/t. The experimental results were examined with performing calculations by a simulation code ESSCAR (Extraction System Simulation Code for Advanced Reprocessing). It was demonstrated that Tc can be almost quantitatively extracted from a dissolver solution and that Tc can also be almost quantitatively recovered by scrubbing. Further, it was clearly presented from the calculation results of ESSCAR that the extraction mechanism of Tc is dominated by the synergistic effect of Zr and U.