Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sato, Hirotaka*; Shiota, Yoshinori*; Morooka, Satoshi; Todaka, Yoshikazu*; Adachi, Nozomu*; Sadamatsu, Sunao*; Oikawa, Kenichi; Harada, Masahide; Zhang, S.*; Su, Y. H.; et al.
Journal of Applied Crystallography, 50(6), p.1601 - 1610, 2017/12
Times Cited Count:17 Percentile:79.13(Chemistry, Multidisciplinary)Mukai, Yoichi*; Nishida, Akemi; Hamamoto, Takuji*; Sakino, Yoshihiro*; Ikawa, Nozomu*; Takeuchi, Yoshitaka*; Chiba, Fumihiko*; Hori, Yoshiro*
Proceedings of 12th International Conference on Shock and Impact Loads on Structures (SI 2017) (USB Flash Drive), p.329 - 338, 2017/06
AIJ guideline against accidental actions is published as a book, "Introduction to shock-resistant design of buildings". This contains respect to objective and scope, design loads, member design, design criteria, non-structural element, progressive collapse and design examples. The objective of AIJ guideline is to minimize human and property damage in building structures against accidental actions based on the performance-based design. Target buildings for design are offices, apartments, hotels, hospitals, schools and public buildings. Structural systems of the buildings are limited to reinforced concrete and steel frame structures. In this paper, the overview of the AIJ guideline is introduced.
Ikawa, Nozomu*; Mukai, Yoichi*; Nishida, Akemi; Hamamoto, Takuji*; Kano, Toshiya*; Ota, Toshiro*; Nakamura, Naohiro*; Komuro, Masato*; Takeuchi, Masato*
Proceedings of 12th International Conference on Shock and Impact Loads on Structures (SI 2017) (USB Flash Drive), p.259 - 268, 2017/06
Accidental actions on building structures involve impact and explosion loads. The design loads due to impact are determined by experiment data, impact simulation and energetics approach. These loads are presented in the form of load-time (F-t) curves caused by collision and explosion. It is assumed that the structure is rigid and immovable and that impacting body absorbs all the energy (i.e., hard impact condition is supposed), because this assumption gives conservative results in general. Responses of individual structural members directly-subjected to an impulsive load are evaluated. These responses are classified into three types; impulsive response, dynamic response, and quasi-static response. The maximum responses are basically estimated by direct integration method with a single-degree-of-freedom (SDOF) model. The procedure of the SDOF modelling based on the classification of types of members and failure modes is proposed in AIJ guideline.
Kamakura, Nozomu; Takeda, Yukiharu; Saito, Yuji; Yamagami, Hiroshi; Tsubota, Masami*; Paik, B.*; Ichikawa, Takayuki*; Kojima, Yoshitsugu*; Muro, Takayuki*; Kato, Yukako*; et al.
Physical Review B, 83(3), p.033103_1 - 033103_4, 2011/01
Times Cited Count:5 Percentile:25.35(Materials Science, Multidisciplinary)The electronic structure of lithium amide, which is lightweight complex hydride expected as a high-capacity hydrogen storage material, is investigated by N 1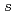 soft X-ray emission spectroscopy (XES) and absorption spectroscopy (XAS). The overall feature of the electronic structure of lithium amide by the XES and XAS is consistent with the band calculation, while the strongly hybridized state with H 1
soft X-ray emission spectroscopy (XES) and absorption spectroscopy (XAS). The overall feature of the electronic structure of lithium amide by the XES and XAS is consistent with the band calculation, while the strongly hybridized state with H 1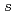 is located at higher binding energy than the band calculation.
is located at higher binding energy than the band calculation.
Maruyama, So; Fujimoto, Nozomu; Kiso, Yoshihiro*; Murakami, Tomoyuki*; Takikawa, Noboru*; *; Sudo, Yukio
JAERI-M 88-154, 147 Pages, 1988/08
no abstracts in English
Kamakura, Nozomu; Takeda, Yukiharu; Saito, Yuji; Yamagami, Hiroshi; Tsubota, Masami*; Paik, B.*; Ichikawa, Takayuki*; Kojima, Yoshitsugu*; Muro, Takayuki*; Kinoshita, Toyohiko*
no journal, ,
Lithium amide (LiNH ) is lightweight complex hydride expected as a high-capacity hydrogen storage material. The electronic structure of lithium amide (LiNH
) is lightweight complex hydride expected as a high-capacity hydrogen storage material. The electronic structure of lithium amide (LiNH ) is investigated by soft X-ray emission spectroscopy (XES) and absorption spectroscopy (XAS). The unoccupied and occupied parts of the N 2
) is investigated by soft X-ray emission spectroscopy (XES) and absorption spectroscopy (XAS). The unoccupied and occupied parts of the N 2 partial density of states are studied by N 1
partial density of states are studied by N 1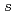 XAS in the fluorescence yield mode and XES using soft X-ray (h
XAS in the fluorescence yield mode and XES using soft X-ray (h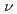 =425eV) of SPring-8. The XES and XAS spectra show a band gap between the valence and conduction bands. The valence band in the XES spectrum consists of three peaks, which extend up to
=425eV) of SPring-8. The XES and XAS spectra show a band gap between the valence and conduction bands. The valence band in the XES spectrum consists of three peaks, which extend up to  -8eV from the valence band top. The overall feature of the electronic structure of lithium amide by the XES and XAS is consistent with the band calculation. The strongly hybridized state with H 1
-8eV from the valence band top. The overall feature of the electronic structure of lithium amide by the XES and XAS is consistent with the band calculation. The strongly hybridized state with H 1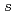 observed on the high binding energy side in the XES is discussed.
observed on the high binding energy side in the XES is discussed.
Kamakura, Nozomu; Takeda, Yukiharu; Okane, Tetsuo; Fujimori, Shinichi; Saito, Yuji; Yamagami, Hiroshi; Miyaoka, Hiroki*; Tsubota, Masami*; Ichikawa, Takayuki*; Kojima, Yoshitsugu*; et al.
no journal, ,
no abstracts in English
Kamakura, Nozomu; Yamagami, Hiroshi; Takeda, Yukiharu; Okane, Tetsuo; Saito, Yuji; Miyaoka, Hiroki*; Tsubota, Masami*; Ichikawa, Takayuki*; Kojima, Yoshitsugu*; Muro, Takayuki*; et al.
no journal, ,
In this research, the electronic states of the insulator alkali metal amide (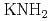 ,
, 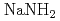 ) and alkaline earth metal amide (
) and alkaline earth metal amide (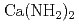 ), which are lightweight complex hydrides being considered as a high-capacity hydrogen storage material, are investigated by the soft X-ray emission (XES)and absorption spectroscopies. The sharp three peak structure commonly observed in the XES spectrum of alkali metal amides shows the localized character of the valence electrons, while the importance of the number of the amide ion is shown by the XES spectrum of
), which are lightweight complex hydrides being considered as a high-capacity hydrogen storage material, are investigated by the soft X-ray emission (XES)and absorption spectroscopies. The sharp three peak structure commonly observed in the XES spectrum of alkali metal amides shows the localized character of the valence electrons, while the importance of the number of the amide ion is shown by the XES spectrum of 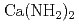 which is clearly different from that of alkali metal amide. The comparison with the band calculation clarifies the electronic sates of
which is clearly different from that of alkali metal amide. The comparison with the band calculation clarifies the electronic sates of 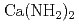 .
.
Kamakura, Nozomu; Takeda, Yukiharu; Yamagami, Hiroshi; Miyaoka, Hiroki*; Tsubota, Masami*; Ichikawa, Takayuki*; Kojima, Yoshitsugu*; Muro, Takayuki*; Kinoshita, Toyohiko*
no journal, ,
Metal amide has been attracted much attention as a lightweight hydride being considered for high capacity hydrogen storage materials. In this research, the electronic states of the insulator alkali metal amide (KNH , NaNH
, NaNH ) and alkaline earth metal amide (Ca(NH
) and alkaline earth metal amide (Ca(NH )
) , Mg(NH
, Mg(NH )
) ) are investigated by the soft X-ray emission spectroscopy (XES) and absorption spectroscopy (XAS) in the total fluorescence yield mode. The localized character of the valence electrons is shown by the sharp three peak structure commonly observed in the XES spectrum of alkali metal amide. The localized character of the valence electrons is shown by the sharp peak structure commonly observed in the XES spectrum of alkali metal amide. The broadening of the N 2p states by the hybridization is observed in the XES spectrum of the alkaline earth metal amide. Decomposition temperature of the metal amide is found to relate to the character of the chemical bond observed in the XAS.
) are investigated by the soft X-ray emission spectroscopy (XES) and absorption spectroscopy (XAS) in the total fluorescence yield mode. The localized character of the valence electrons is shown by the sharp three peak structure commonly observed in the XES spectrum of alkali metal amide. The localized character of the valence electrons is shown by the sharp peak structure commonly observed in the XES spectrum of alkali metal amide. The broadening of the N 2p states by the hybridization is observed in the XES spectrum of the alkaline earth metal amide. Decomposition temperature of the metal amide is found to relate to the character of the chemical bond observed in the XAS.