Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ouchi, Kazuki; Komatsu, Atsushi; Takao, Koichiro*; Kitatsuji, Yoshihiro; Watanabe, Masayuki
Chemistry Letters, 50(6), p.1169 - 1172, 2021/06
Times Cited Count:2 Percentile:14.19(Chemistry, Multidisciplinary)The electrochemical behavior of uranium (IV) tetrachloride in ionic liquid-DMF mixture was studied for first time in order to build a redox flow battery (RFB) using U as an electrode active material. We found a quasi-reversible U /U
/U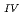 couple that could be applied to the anode reaction of the RFB.
couple that could be applied to the anode reaction of the RFB.
Komatsu, Masayuki*; Zhao, D.*
JNC TJ7400 2005-053, 40 Pages, 2003/03
This report presents general characteristics of the fracture systems in granitic rocks on the basis of actual field data and development of field investigation techniques for fracture surveys.
Komatsu, Masayuki*
JNC TJ7400 2005-050, 45 Pages, 2002/03
This report presents general characteristics of the fracture systems in granitic rocks on the basis of actual field data and development of field investigation techniques for fracture surveys.
Komatsu, Junji*; *; Seki, Masayuki; *; Tobita, Noriyuki
PNC TN845 85-09, 57 Pages, 1985/09
Halden IFA-554/555 irradiation test is planned to examine the fuel behavier of ATR type fuel under load follow reactor operation. Eight fuel rods for this irradiation test were fabricated in Plutonium Fuel Div. in Tokai Works PNC. These fuel rods install instruments for the measurement of fuel columnelongation, cladding elongation, plenum pressure and fuel center temperature. The fuel pellets were made from one type of PuO2 raw materials. It was obtained by precipitation directde nitration process . The fuel compositionis 4.4% PuO2-UO2(natural), and the pellet nominal dimension is 12.40 mm diameter and 13 mm height.
Ouchi, Kazuki; Komatsu, Atsushi; Takao, Koichiro*; Kitatsuji, Yoshihiro; Watanabe, Masayuki
no journal, ,
In this study, the electrochemical behavior of U(IV) in ionic liquid-DMF mixtures was investigated. DMF was added to the ionic liquid to reduce the viscosity to develop the redox flow battery (RFB) based on the reactions of U. The voltammogram of UCl was measured. Redox waves at -1.6 and -1.5 V were observed based on the reaction of U(III)/U(IV). Further oxidation current of U(IV) was not observed at more negative potential than Cl
was measured. Redox waves at -1.6 and -1.5 V were observed based on the reaction of U(III)/U(IV). Further oxidation current of U(IV) was not observed at more negative potential than Cl oxidation. We conclude that U(III)/U(IV) in ionic liquid-DMF mixture is applicable to the cathode reaction of RFB from these results.
oxidation. We conclude that U(III)/U(IV) in ionic liquid-DMF mixture is applicable to the cathode reaction of RFB from these results.
Ouchi, Kazuki; Komatsu, Atsushi; Takao, Koichiro*; Kitatsuji, Yoshihiro; Watanabe, Masayuki
no journal, ,
The electrode reaction of uranium (IV) tetrachloride in an ionic liquid-DMF mixture was studied in order to build a redox flow battery (RFB) using U as an electrode active material. We found the quasi-reversible U(III)/U(IV) reaction that the formal potential is almost constant at -1.559  0.003 V. This reaction could be applied to the negative electrode reaction of the RFB.
0.003 V. This reaction could be applied to the negative electrode reaction of the RFB.