Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Abe, Takeyasu; Kuribayashi, Takahiro*; Nakamura, Michihiko*
European Journal of Mineralogy, 29(6), p.949 - 957, 2017/12
Times Cited Count:2 Percentile:6.21(Mineralogy)Polarised infrared spectra of synthetic single crystals and radiation-damaged natural samples were collected to examine hydroxyl incorporation in monazite. The IR spectra of pure synthetic monazite contain two OH stretching bands at 3163 and 3335 cm with contrasting bandwidths of 40 and 90 cm
with contrasting bandwidths of 40 and 90 cm , respectively. The two OH bands show strong pleochroism and dominant infrared absorption in the Y direction. The IR spectra of natural monazite contains a weak pleochroic OH band centered around 3400 cm
, respectively. The two OH bands show strong pleochroism and dominant infrared absorption in the Y direction. The IR spectra of natural monazite contains a weak pleochroic OH band centered around 3400 cm with a bandwidth of more than 200 cm
with a bandwidth of more than 200 cm . During step-heating experiments, this broad OH band split into several bands, and these bands differ from those observed in the spectra of synthetic samples. The OH stretching signals in the spectra of both natural and synthetic samples disappeared after heating at 1000
. During step-heating experiments, this broad OH band split into several bands, and these bands differ from those observed in the spectra of synthetic samples. The OH stretching signals in the spectra of both natural and synthetic samples disappeared after heating at 1000 C. Based on these results, OH defects in natural monazite arise because of secondary hydration facilitated by radiation damage, as in the case of natural zircon and xenotime.
C. Based on these results, OH defects in natural monazite arise because of secondary hydration facilitated by radiation damage, as in the case of natural zircon and xenotime.
 -AlOOH by using single-crystal synchrotron X-ray diffraction method
-AlOOH by using single-crystal synchrotron X-ray diffraction methodKuribayashi, Takahiro*; Sano, Asami; Nagase, Toshiro*
Physics and Chemistry of Minerals, 41(4), p.303 - 312, 2014/04
Times Cited Count:38 Percentile:79.07(Materials Science, Multidisciplinary)Pressure-induced phase transition of  -AlOOH was confirmed between 6.1 and 8.2 GPa by using a single-crystal synchrotron X-ray diffraction method. The phase transition is reversible and unquenchable. The space group of the post-transition phase should be
-AlOOH was confirmed between 6.1 and 8.2 GPa by using a single-crystal synchrotron X-ray diffraction method. The phase transition is reversible and unquenchable. The space group of the post-transition phase should be 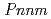 or
or 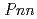 2 to satisfy the systematic absence rule. Crystal structure refinements of the post-transition phase conducted for the three models (
2 to satisfy the systematic absence rule. Crystal structure refinements of the post-transition phase conducted for the three models (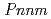 ,
, 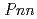 2, and
2, and  2
2
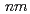 ) indicate that the space group of the post-transition phase is
) indicate that the space group of the post-transition phase is 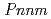 . The O-O distance of hydrogen bond in the post-transition phase at 8.2 GPa is 2.439(6)
. The O-O distance of hydrogen bond in the post-transition phase at 8.2 GPa is 2.439(6)  and is significantly longer than the predicted distance (2.366
and is significantly longer than the predicted distance (2.366  ) of the hydrogen bond symmetrization in
) of the hydrogen bond symmetrization in  -AlOOH. The H distribution in the post-transition phase would display a fully disordered hydrogen bond pattern.
-AlOOH. The H distribution in the post-transition phase would display a fully disordered hydrogen bond pattern.
Sano, Asami; Kuribayashi, Takahiro*; Komatsu, Kazuki*; Yagi, Takehiko*; Otani, Eiji*
Physics of the Earth and Planetary Interiors, 189(1-2), p.56 - 62, 2011/11
Times Cited Count:22 Percentile:52.77(Geochemistry & Geophysics)A neutron powder diffraction experiment was conducted to refine the hydrogen position of Mg-endmember deuterated wadsleyite. Preliminary refinement using the dry-structure determined by single crystal X-ray diffraction reveals a maximum peak Q1 of nuclear density in the difference Fourier map at the M3 octahedral edge, between the O1 and O4 atoms. Full Rietveld refinement was conducted assuming that the maximum peak corresponds to deuterium atom. The deuterium position was determined as (0.096, 0.289, 0.315) with occupancy of 8.2%. The structure determined by this study predicts that the hydration of wadsleyite has an anisotropic effect on diffusion properties.
Haba, Hiromitsu*; Akiyama, Takahiro*; Kaji, Daiya*; Kikunaga, Hidetoshi*; Kuribayashi, Takahiro*; Morimoto, Koji*; Morita, Kosuke*; Oe, Kazuhiro*; Sato, Nozomi*; Shinohara, Atsushi*; et al.
European Physical Journal D, 45(1), p.81 - 86, 2007/10
Times Cited Count:11 Percentile:49.57(Optics)A review is given on the startup of the superheavy element (SHE) chemistry at RIKEN. A gas-jet transport system for the SHE chemistry has been coupled to the gas-filled recoil ion separator GARIS at the RIKEN Linear Accelerator. The performance of the system was appraised using  Fr and
Fr and 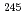 Fm produced in the
Fm produced in the 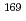 Tm(
Tm(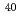 Ar,3
Ar,3 )
) Fr and
Fr and  Pb(
Pb(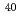 Ar,3
Ar,3 )
)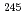 Fm reactions, respectively. The
Fm reactions, respectively. The  particles of
particles of  Fr and
Fr and 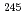 Fm separated with GARIS and transported by the gas-jet were identified with a rotating wheel system for
Fm separated with GARIS and transported by the gas-jet were identified with a rotating wheel system for  spectrometry under desired low background condition. The high gas-jet efficiencies over 80% were independent of the beam intensities up to 2 particle
spectrometry under desired low background condition. The high gas-jet efficiencies over 80% were independent of the beam intensities up to 2 particle  A. A gas-jet coupled target system for the production of SHEs was also installed on the beam line of the RIKEN K70 AVF cyclotron. The gas-jet transport of
A. A gas-jet coupled target system for the production of SHEs was also installed on the beam line of the RIKEN K70 AVF cyclotron. The gas-jet transport of  No and
No and  Rf produced in the
Rf produced in the  U(
U( Ne,5
Ne,5 )
) No and
No and  Cm(
Cm( O,5
O,5 )
) Rf reactions, respectively, was conducted for the future chemical studies of
Rf reactions, respectively, was conducted for the future chemical studies of  Sg via the
Sg via the  Cm(
Cm( Ne, 5
Ne, 5 )
) Sg reaction.
Sg reaction.
Toyoshima, Atsushi; Kasamatsu, Yoshitaka; Tsukada, Kazuaki; Haba, Hiromitsu*; Asai, Masato; Ishii, Yasuo; Tome, Hayato; Sato, Tetsuya; Nishinaka, Ichiro; Nagame, Yuichiro; et al.
no journal, ,
no abstracts in English
 media
mediaKasamatsu, Yoshitaka; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Haba, Hiromitsu*; Ishii, Yasuo; Tome, Hayato; Nishinaka, Ichiro; Akiyama, Kazuhiko*; Kikunaga, Hidetoshi*; et al.
no journal, ,
Dubnium-262 was produced in the  Cm(
Cm( F, 5
F, 5 ) reaction at the JAEA tandem accelerator. Adsorption of Db on the anion-exchange resin was investigated in 0.89 M HF/0.3 M HNO
) reaction at the JAEA tandem accelerator. Adsorption of Db on the anion-exchange resin was investigated in 0.89 M HF/0.3 M HNO solution. The anion-exchange behavior of Nb, Ta, and Pa as homologues of Db was also examined in details in HF/HNO
solution. The anion-exchange behavior of Nb, Ta, and Pa as homologues of Db was also examined in details in HF/HNO solutions. From the comparison of those results, we found that the adsorption of Db on the anion-exchange resin is considerably weaker than that of Ta and is relatively similar to those of Nb and Pa in the studied conditions.
solutions. From the comparison of those results, we found that the adsorption of Db on the anion-exchange resin is considerably weaker than that of Ta and is relatively similar to those of Nb and Pa in the studied conditions.
 solutions
solutionsKasamatsu, Yoshitaka; Tome, Hayato; Toyoshima, Atsushi; Tsukada, Kazuaki; Asai, Masato; Ishii, Yasuo; Nishinaka, Ichiro; Sato, Tetsuya; Shinohara, Nobuo; Nagame, Yuichiro; et al.
no journal, ,
Anion-exchange behavior of  Db (half-life
Db (half-life 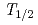 = 34 s) produced in the
= 34 s) produced in the  Cm(
Cm( F, 5
F, 5 ) reaction at the JAEA tandem accelerator was investigated in the mixed 0.89 M HF/0.3 M HNO
) reaction at the JAEA tandem accelerator was investigated in the mixed 0.89 M HF/0.3 M HNO solution ([F
solution ([F ] = 3
] = 3  10
10 M) with the automated ion-exchange separation apparatus coupled with the detection system for alpha-spectroscopy (AIDA). Anion-exchange behavior of its lighter homologues, Nb and Ta, was also studied under the same conditions using
M) with the automated ion-exchange separation apparatus coupled with the detection system for alpha-spectroscopy (AIDA). Anion-exchange behavior of its lighter homologues, Nb and Ta, was also studied under the same conditions using  Nb (
Nb (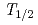 = 14.3 min) and
= 14.3 min) and 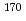 Ta (
Ta (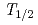 = 6.76 min) produced in the
= 6.76 min) produced in the 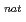 Ge(
Ge( F,
F, 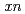 ) and
) and 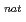 Gd(
Gd( F,
F, 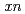 ) reactions, respectively. It was found that the adsorption probability on the anion-exchange resin is in the order of Ta
) reactions, respectively. It was found that the adsorption probability on the anion-exchange resin is in the order of Ta  Nb
Nb  Db under the present condition.
Db under the present condition.
 solutions
solutionsKasamatsu, Yoshitaka; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Haba, Hiromitsu*; Ishii, Yasuo; Tome, Hayato; Nishinaka, Ichiro; Akiyama, Kazuhiko*; Kikunaga, Hidetoshi*; et al.
no journal, ,
Dubnium-262 was produced in the nuclear reaction of  Cm(
Cm( F,5n)
F,5n) Db using the JAEA Tandem accelerator. The reaction products were rapidly transported to the chemistry laboratory and the anion-exchange behavior of Db in HF/HNO
Db using the JAEA Tandem accelerator. The reaction products were rapidly transported to the chemistry laboratory and the anion-exchange behavior of Db in HF/HNO media was investigated with an on-line rapid ion-exchange apparatus. Based on the comparison of the behavior of Db with that of its homologues (Nb, Ta, and Pa), the fluoride complex formation of Db was discussed.
media was investigated with an on-line rapid ion-exchange apparatus. Based on the comparison of the behavior of Db with that of its homologues (Nb, Ta, and Pa), the fluoride complex formation of Db was discussed.
Sano, Asami; Yagi, Takehiko*; Komatsu, Kazuki*; Kuribayashi, Takahiro*
no journal, ,
no abstracts in English
Sano, Asami; Kuribayashi, Takahiro*; Komatsu, Kazuki*; Yagi, Takehiko*; Otani, Eiji*
no journal, ,
Wadsleyite is a main constituent of upper part of the mantle transition zone, at 410 - 520 km depth of the earth. Since the Smyth's prediction, wadsleyite, which is one of the nominally anhydrous mineral (NAMs) have been attracted as a host of water in the mantle. Subsequent studies have also revealed that a small amount of hydrogen cause changes of some physical properties of wadsleyite dramatically. To understand the effect of hydration of wadsleyite on its physical properties, it is essential to know the position of hydrogen in the structure. In this study, we intend to determine the hydrogen position of wadsleyite by neutron powder diffraction experiment. Full Rietveld refinement with deuterium was successfully conducted, which revealed that an O-D bond is formed at the edge of the M3 octahedron between the O1...O4 (3.074 A).
Sano, Asami; Komatsu, Kazuki*; Kuribayashi, Takahiro*; Yagi, Takehiko*; Otani, Eiji*
no journal, ,
New neutron diffraction beamline for high pressure experiment is under construction at MLF, J-PARC and will be available soon. In this paper, I will present examples of neutron diffraction studies on hydrous and nominally anhydrous minerals to show a potential utility of neutron diffraction in the investigation of high pressure mineral physics. (1) Emboss hydrogen in mineral; Neutron scattering length of hydrogen (deuterium) is sufficient compared to the main constituent of minerals, thus neutron diffraction is a powerful tool to investigate the hydrogen in minerals. I will present the result of neutron diffraction study on nominally anhydrous mineral of wadsleyite. (2) Distinguish Isotopes; Another characteristic property of neutron scattering is an ability to distinguish isotopes. Neutron diffraction on  -AlOOH and
-AlOOH and  -AlOOD shows the strong isotope effects on the geometry of hydrogen bond.
-AlOOD shows the strong isotope effects on the geometry of hydrogen bond.
Sano, Asami; Nagai, Takaya*; Iizuka, Riko*; Seto, Yusuke*; Kuribayashi, Takahiro*; Hattori, Takanori
no journal, ,
Lawsonite is a hydrous mineral which is considered as a main carrier of hydrogen in the subtucting slab. Previous single crystal X-ray diffraction and neutron diffraction studies indicate that there exist two phase transitions at low temperature. A property of low temperature is sometimes considered to be equivalent to the behavior at high pressure, and some studies pointed out the possibility of transition at high pressure. To investigate the pressure response of hydrogen bond and phase transition in lawsonite, neutron diffraction experiment was conducted. High pressure and high temperature neutron diffraction experiment was conducted by 6-ram press at J-PARC MLF. Using 6-6 type anvil with TEL size of 10 mm, neutron diffraction pattern was corrected up to 6 GPa and 800 C. In addition, hydrostatic experiment at ambient pressure was conducted using Paris-Edinburgh press. New peak was observed at 1.83
C. In addition, hydrostatic experiment at ambient pressure was conducted using Paris-Edinburgh press. New peak was observed at 1.83 that indicates phase transition at high pressure.
that indicates phase transition at high pressure.
 monazite; Investigation of impurity incorporation into interstitial site
monazite; Investigation of impurity incorporation into interstitial siteAbe, Takeyasu; Kuribayashi, Takahiro*; Nakamura, Michihiko*
no journal, ,
no abstracts in English