Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yakushev, A.*; Lens, L.*; D llmann, Ch. E.*; Khuyagbaatar, J.*; J
llmann, Ch. E.*; Khuyagbaatar, J.*; J ger, E.*; Krier, J.*; Runke, J.*; Albers, H. M.*; Asai, Masato; Block, M.*; et al.
ger, E.*; Krier, J.*; Runke, J.*; Albers, H. M.*; Asai, Masato; Block, M.*; et al.
Frontiers in Chemistry (Internet), 10, p.976635_1 - 976635_11, 2022/08
Times Cited Count:9 Percentile:79.28(Chemistry, Multidisciplinary)Flerovium (Fl, element 114) is the heaviest element chemically studied so far. The first chemical experiment on Fl suggested that Fl is a noble-gas-like element, while the second studies suggested that Fl has a volatile-metal-like character. To obtain more reliable conclusion, we performed further experimental studies on Fl adsorption behavior on Si oxide and gold surfaces. The present results suggest that Fl is highly volatile and less reactive than the volatile metal, Hg, but has higher reactivity than the noble gas, Rn.
Khuyagbaatar, J.*; Yakushev, A.*; D llmann, Ch. E.*; Ackermann, D.*; Andersson, L.-L.*; Asai, Masato; Block, M.*; Boll, R. A.*; Brand, H.*; Cox, D. M.*; et al.
llmann, Ch. E.*; Ackermann, D.*; Andersson, L.-L.*; Asai, Masato; Block, M.*; Boll, R. A.*; Brand, H.*; Cox, D. M.*; et al.
Physical Review C, 102(6), p.064602_1 - 064602_9, 2020/12
Times Cited Count:43 Percentile:98.01(Physics, Nuclear)A search for production of the superheavy elements with atomic numbers 119 and 120 was performed in the  Ti+
Ti+ Bk and
Bk and  Ti+
Ti+ Cf fusion-evaporation reactions, respectively, at the gas-filled recoil separator TASCA. Over four months of irradiation, neither was detected at cross-section sensitivity levels of 65 and 200 fb, respectively. The non-observation of elements 119 and 120 is discussed within the concept of fusion-evaporation reactions including various theoretical predictions on the fission-barrier heights of superheavy nuclei in the region of the island of stability.
Cf fusion-evaporation reactions, respectively, at the gas-filled recoil separator TASCA. Over four months of irradiation, neither was detected at cross-section sensitivity levels of 65 and 200 fb, respectively. The non-observation of elements 119 and 120 is discussed within the concept of fusion-evaporation reactions including various theoretical predictions on the fission-barrier heights of superheavy nuclei in the region of the island of stability.
 Ca+
Ca+ Bk leading to formation of the element Ts (Z=117)
Bk leading to formation of the element Ts (Z=117)Khuyagbaatar, J.*; Yakushev, A.*; D llmann, Ch. E.*; Ackermann, D.*; Andersson, L.-L.*; Asai, Masato; Block, M.*; Boll, R. A.*; Brand, H.*; Cox, D. M.*; et al.
llmann, Ch. E.*; Ackermann, D.*; Andersson, L.-L.*; Asai, Masato; Block, M.*; Boll, R. A.*; Brand, H.*; Cox, D. M.*; et al.
Physical Review C, 99(5), p.054306_1 - 054306_16, 2019/05
Times Cited Count:23 Percentile:90.91(Physics, Nuclear)We have performed an experiment to synthesize the element 117 (Ts) with the  Ca+
Ca+ Bk fusion reaction. Four
Bk fusion reaction. Four  -decay chains attributed to the element 117 were observed. Two of them were long decay chains which can be assigned to the one originating from the
-decay chains attributed to the element 117 were observed. Two of them were long decay chains which can be assigned to the one originating from the  decay of
decay of 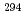 Ts. The other two were short decay chains which are consistent with the one originating from the
Ts. The other two were short decay chains which are consistent with the one originating from the  decay of
decay of 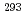 Ts. We have compared the present results with the literature data, and found that our present results mostly confirmed the literature data, leading to the firm confirmation of the synthesis of the element 117.
Ts. We have compared the present results with the literature data, and found that our present results mostly confirmed the literature data, leading to the firm confirmation of the synthesis of the element 117.
 and Au surfaces in preparation for chemical investigations on Cn, Nh, and Fl at TASCA
and Au surfaces in preparation for chemical investigations on Cn, Nh, and Fl at TASCALens, L.*; Yakushev, A.*; D llmann, Ch. E.*; Asai, Masato; Ballof, J.*; Block, M.*; David, H. M.*; Despotopulos, J.*; Di Nitto, A.*; Eberhardt, K.*; et al.
llmann, Ch. E.*; Asai, Masato; Ballof, J.*; Block, M.*; David, H. M.*; Despotopulos, J.*; Di Nitto, A.*; Eberhardt, K.*; et al.
Radiochimica Acta, 106(12), p.949 - 962, 2018/12
Times Cited Count:8 Percentile:62.99(Chemistry, Inorganic & Nuclear)Online gas-solid adsorption studies with single atom quantities of Hg, Tl, and Pb on SiO and Au surfaces were carried out using short-lived radioisotopes with half-lives in the range of 4-49 s. This is a model study to measure adsorption enthalpies of superheavy elements Cn, Nh, and Fl. The short-lived isotopes were produced and separated by the gas-filled recoil separator TASCA at GSI. The products were stopped in He gas, and flushed into gas chromatography columns made of Si detectors whose surfaces were covered by SiO
and Au surfaces were carried out using short-lived radioisotopes with half-lives in the range of 4-49 s. This is a model study to measure adsorption enthalpies of superheavy elements Cn, Nh, and Fl. The short-lived isotopes were produced and separated by the gas-filled recoil separator TASCA at GSI. The products were stopped in He gas, and flushed into gas chromatography columns made of Si detectors whose surfaces were covered by SiO or Au. The short-lived Tl and Pb were successfully measured by the Si detectors with the SiO
or Au. The short-lived Tl and Pb were successfully measured by the Si detectors with the SiO surface at room temperature. On the other hand, the Hg did not adsorb on the SiO
surface at room temperature. On the other hand, the Hg did not adsorb on the SiO surface, but adsorbed on the Au surface. The results demonstrated that the adsorption properties of short-lived Hg, Tl, and Pb could be studied with this setup, and that this method is applicable to the experiment for Cn, Nh, and Fl.
surface, but adsorbed on the Au surface. The results demonstrated that the adsorption properties of short-lived Hg, Tl, and Pb could be studied with this setup, and that this method is applicable to the experiment for Cn, Nh, and Fl.
Even, J.*; Ackermann, D.*; Asai, Masato; Block, M.*; Brand, H.*; Di Nitto, A.*; D llmann, Ch. E.*; Eichler, R.*; Fan, F.*; Haba, Hiromitsu*; et al.
llmann, Ch. E.*; Eichler, R.*; Fan, F.*; Haba, Hiromitsu*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(3), p.2457 - 2466, 2015/03
Times Cited Count:15 Percentile:77.56(Chemistry, Analytical)Rapid In situ synthesis of metal carbonyl complexes has been demonstrated using short-lived isotopes produced in nuclear fission and fusion reactions. The short-lived isotopes with high recoil energy directly react with carbon-monoxides and form carbonyl complexes. Only highly volatile complexes were fast transported in a gas stream to counting and chemistry devices. Short-lived Mo, Tc, Ru, Rh, W, Re, Os, and Ir were found to form volatile carbonyl complexes, while no volataile complex of Hf and Ta were detected. This technique has been applied to a chemical investigation of the superheavy element Sg (atomic number 106), and will be applicable to various fields of nuclear science with short-lived transition metal isotopes.
 Ca +
Ca +  Bk fusion reaction leading to element Z = 117; Long-lived
Bk fusion reaction leading to element Z = 117; Long-lived  -decaying
-decaying 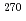 Db and discovery of
Db and discovery of 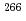 Lr
LrKhuyagbaatar, J.*; Yakushev, A.*; D llmann, Ch. E.*; Ackermann, D.*; Andersson, L.-L.*; Asai, Masato; Block, M.*; Boll, R. A.*; Brand, H.*; Cox, D. M.*; et al.
llmann, Ch. E.*; Ackermann, D.*; Andersson, L.-L.*; Asai, Masato; Block, M.*; Boll, R. A.*; Brand, H.*; Cox, D. M.*; et al.
Physical Review Letters, 112(17), p.172501_1 - 172501_5, 2014/05
Times Cited Count:208 Percentile:98.43(Physics, Multidisciplinary)The superheavy element with atomic number 117 was produced in the  Ca +
Ca +  Bk fusion reaction using the gas-filled recoil separator TASCA at GSI in Germany. This result verified the previous result of the discovery of new element 117 reported by Flerov Laboratory of Nuclear Reactions in Russia, which makes certain the synthesis and discovery of element 117 in human history. On the other hand, the last nucleus in the
Bk fusion reaction using the gas-filled recoil separator TASCA at GSI in Germany. This result verified the previous result of the discovery of new element 117 reported by Flerov Laboratory of Nuclear Reactions in Russia, which makes certain the synthesis and discovery of element 117 in human history. On the other hand, the last nucleus in the  decay chain from the element 117 was assigned to be the unknown nucleus
decay chain from the element 117 was assigned to be the unknown nucleus 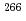 Lr instead of the previously reported
Lr instead of the previously reported 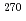 Db, and
Db, and 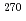 Db was found to be the
Db was found to be the  -decaying nucleus with very long half-life.
-decaying nucleus with very long half-life.