Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sasaki, Hirokazu*; Nishikubo, Hideo*; Nishida, Shinsuke*; Yamazaki, Satoshi*; Nakasaki, Ryusuke*; Isomatsu, Takemi*; Minato, Ryuichiro*; Kinugawa, Kohei*; Imamura, Akihiro*; Otomo, Shinya*; et al.
Furukawa Denko Jiho, (138), p.2 - 10, 2019/02
no abstracts in English
Hayashi, Hirokazu; Akabori, Mitsuo; Minato, Kazuo
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1331 - 1334, 2015/02
Times Cited Count:0 Percentile:0.01(Chemistry, Analytical)Electrochemical behavior of Am in NaCl-2CsCl melt at 823 K was investigated by transient electrochemical techniques such as cyclic voltammetry and differential pulse voltammetry. The results show that Am(III) ion is reduced to Am metal by a two-step mechanism via Am(II) ion. Formal standard potential of Am(III)/Am(II) and that of Am(II)/Am(0) redox couples have been determined to be -2.73 and -2.97 V vs Cl /Cl
/Cl , respectively.
, respectively.
Takano, Masahide; Hayashi, Hirokazu; Minato, Kazuo
Journal of Nuclear Materials, 448(1-3), p.66 - 71, 2014/05
Times Cited Count:2 Percentile:16.44(Materials Science, Multidisciplinary)A powder sample of curium nitride (CmN) containing 0.35%-PuN and 3.59%-AmN was prepared by carbothermic nitridation of the oxide. The lattice expansion induced by self-irradiation damage at room temperature was measured as a function of time. The saturated  a/a
a/a value was 0.43%, which is greater than those for transuranium dioxides available in literature. The undamaged lattice parameter at 297
value was 0.43%, which is greater than those for transuranium dioxides available in literature. The undamaged lattice parameter at 297 1 K was determined to be 0.50261
1 K was determined to be 0.50261 0.00006 nm. Temperature dependence of the lattice parameter was measured by a high temperature X-ray diffractometer in the temperature range up to 1375 K. The linear thermal expansion of the lattice from 293 to 1273 K is 0.964% and the corresponding thermal expansion coefficient is 9.84
0.00006 nm. Temperature dependence of the lattice parameter was measured by a high temperature X-ray diffractometer in the temperature range up to 1375 K. The linear thermal expansion of the lattice from 293 to 1273 K is 0.964% and the corresponding thermal expansion coefficient is 9.84  10
10 K
K . Comparing with the other actinide nitrides, it was found that CmN lies between the higher expansion nitrides (PuN and AmN) and the lower expansion nitrides (UN and NpN).
. Comparing with the other actinide nitrides, it was found that CmN lies between the higher expansion nitrides (PuN and AmN) and the lower expansion nitrides (UN and NpN).
Kugo, Teruhiko; Ishikawa, Makoto; Nagaya, Yasunobu; Yokoyama, Kenji; Fukaya, Yuji; Maruyama, Hiromi*; Ishii, Yoshihiko*; Fujimura, Koji*; Kondo, Takao*; Minato, Hirokazu*; et al.
JAEA-Research 2013-046, 53 Pages, 2014/03
The present report summarizes the results of a 2-year cooperative study between JAEA and Hitachi-GE in order to contribute to the settlement of the Fukushima-Daiichi Nuclear Power Plants which suffered from the severe accident on March 2011. In the present study, the possible scenarios to reach the recriticality events in Fukushima-Daiichi were investigated first. Then, the analytical methodology to evaluate the time-dependent recriticality events has been developed by modelling the reactivity insertion rate and the possible feedback according to the recriticality scenarios identified in the first step. The methodology developed here has been equipped as a transient simulation tool, PORCAS, which is operated on a multi-purpose platform for reactor analysis, MARBLE. Finally, the radiation exposure rates by the postulated recriticality events in Fukushima-Daiichi were approximately evaluated to estimate the impact to the public environment.
Hayashi, Hirokazu; Takano, Masahide; Kurata, Masaki; Minato, Kazuo
Journal of Nuclear Materials, 440(1-3), p.477 - 479, 2013/09
Times Cited Count:5 Percentile:38.62(Materials Science, Multidisciplinary)Neptunium trichloride of high purity was synthesized by the solid-state reaction of neptunium nitride, which was prepared from the oxide by the carbothermic reduction method, and cadmium chloride in a similar manner as reported for synthesis of AmCl . Lattice parameters of hexagonal NpCl
. Lattice parameters of hexagonal NpCl were determined from the X-ray diffraction pattern to be a = 0.7421
were determined from the X-ray diffraction pattern to be a = 0.7421  0.0006 nm and c = 0.4268
0.0006 nm and c = 0.4268  0.0003 nm, which fairly agree with the reported values (a = 0.742
0.0003 nm, which fairly agree with the reported values (a = 0.742  0.001 nm and c = 0.4281
0.001 nm and c = 0.4281  0.0005 nm). Melting temperature of NpCl
0.0005 nm). Melting temperature of NpCl was measured with about 1 mg of the sample which was hermetically encapsulated in a gold crucible using a differential thermal analyzer with heating and cooling rate of 10 K/min in an argon gas flow (50 mL/min). The melting temperature of NpCl
was measured with about 1 mg of the sample which was hermetically encapsulated in a gold crucible using a differential thermal analyzer with heating and cooling rate of 10 K/min in an argon gas flow (50 mL/min). The melting temperature of NpCl was determined to 1070
was determined to 1070  3 K, which is close to the recommended value 1075
3 K, which is close to the recommended value 1075 30 K, which was derived from the mean value of the melting temperature for UCl
30 K, which was derived from the mean value of the melting temperature for UCl (1115K) and that for PuCl
(1115K) and that for PuCl (1041 K).
(1041 K).
Hayashi, Hirokazu; Hagiya, Hiromichi; Kim, S.-Y.*; Morita, Yasuji; Akabori, Mitsuo; Minato, Kazuo
Journal of Radioanalytical and Nuclear Chemistry, 296(3), p.1275 - 1286, 2013/06
Times Cited Count:4 Percentile:32.48(Chemistry, Analytical) Cm was separated and recovered as an oxalate from
Cm was separated and recovered as an oxalate from  Cm-
Cm- Pu mixed oxide which had been
Pu mixed oxide which had been  Cm oxide sample prepared 40 years ago. Plutonium ions were removed from the solution prepared by dissolution of
Cm oxide sample prepared 40 years ago. Plutonium ions were removed from the solution prepared by dissolution of  Cm-
Cm- Pu mixed oxide in nitric acid, by using an anion exchange resin column. Curium oxalate, a precursor compound of curium oxide, was prepared from the purified curium solution and supplied for the syntheses and measurements of the thermochemical properties of curium compounds.
Pu mixed oxide in nitric acid, by using an anion exchange resin column. Curium oxalate, a precursor compound of curium oxide, was prepared from the purified curium solution and supplied for the syntheses and measurements of the thermochemical properties of curium compounds.
Osaka, Masahiko; Konashi, Kenji*; Hayashi, Hirokazu; Li, D.*; Homma, Yoshiya*; Yamamura, Tomoo*; Sato, Isamu; Miwa, Shuhei; Sekimoto, Shun*; Kubota, Takumi*; et al.
Proceedings of International Conference on Toward and Over the Fukushima Daiichi Accident (GLOBAL 2011) (CD-ROM), 5 Pages, 2011/12
Summer schools for future experts have successfully been completed under Japan Actinide Network (J-ACTINET) for the purpose of development of human resources who are expected to be engaged in every areas of actinide-research/engineering. The first summer school was held in Ibaraki-area in August 2009, followed by the second one in Kansai-area in August 2010. Two summer schools have focused on actual experiences of actinides in actinide-research fields for university students and young researchers/engineers as an introductory course of actinide-researches. Several quasi actinide-handling experiences at the actinide-research fields have attracted attentions of participants at the first school in Ibaraki-area. The actual experiments using actinides-containing solutions have been carried out at the second school in Kansai-area. Future summer schools will be held every year for the sustainable human resource development in various actinide-research fields.
 )
) and CaCl
and CaCl
Fujii, Toshiyuki*; Okude, Genki*; Uehara, Akihiro*; Sekimoto, Shun*; Hayashi, Hirokazu; Akabori, Mitsuo; Minato, Kazuo; Yamana, Hajimu*
Journal of Radioanalytical and Nuclear Chemistry, 288(1), p.181 - 187, 2011/04
Times Cited Count:6 Percentile:44.28(Chemistry, Analytical)Distribution behavior of Ce(III), Am(III), and Cm(III) between tri-n-butyl phosphate solution and molten calcium nitrate hydrate Ca(NO )
)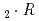 H
H O or molten calcium chloride hydrate CaCl
O or molten calcium chloride hydrate CaCl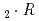 H
H O was studied radiochemically. In Ca(NO
O was studied radiochemically. In Ca(NO )
)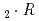 H
H O systems, maximum separation factors of Ce and Cm to Am were observed to be 12 (Ce/Am) and 1.7 (Cm/Am). The distribution ratios of these elements increased with the decrease of water activity in the hydrates, and the extractabilities at the water deficient region was less sensitive compared to those at the water abundant region. This trend was similar to the coordination circumstance change observed in electronic absorption spectra of Nd(III) in the hydrates.
O systems, maximum separation factors of Ce and Cm to Am were observed to be 12 (Ce/Am) and 1.7 (Cm/Am). The distribution ratios of these elements increased with the decrease of water activity in the hydrates, and the extractabilities at the water deficient region was less sensitive compared to those at the water abundant region. This trend was similar to the coordination circumstance change observed in electronic absorption spectra of Nd(III) in the hydrates.
Hayashi, Hirokazu; Akabori, Mitsuo; Arai, Yasuo; Minato, Kazuo
Proceedings of 10th OECD/NEA Information Exchange Meeting on Actinide and Fission Product Partitioning and Transmutation (CD-ROM), 9 Pages, 2010/00
R&D on the transmutation of long-lived minor actinides (MA) by the accelerator-driven system (ADS) using nitride fuels is underway at JAEA. In regard to reprocessing technology, pyrochemical process has several advantages in case of treating spent fuel with large decay heat and fast neutron emission, and recovering highly enriched 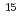 N. In the pyrochemical reprocessing, plutonium and MA are dissolved in LiCl-KCl eutectic melts and selectively recovered into liquid cadmium cathode by molten salt electrorefining. The electrochemical behavior in LiCl-KCl eutectic melts and the subsequent nitride formation behavior of plutonium and MA recovered in liquid Cd cathode are investigated. Recent results on the behavior of americium (Am) in the pyrochemical process for the treatment of spent nitride fuel, which include preparation of AmCl
N. In the pyrochemical reprocessing, plutonium and MA are dissolved in LiCl-KCl eutectic melts and selectively recovered into liquid cadmium cathode by molten salt electrorefining. The electrochemical behavior in LiCl-KCl eutectic melts and the subsequent nitride formation behavior of plutonium and MA recovered in liquid Cd cathode are investigated. Recent results on the behavior of americium (Am) in the pyrochemical process for the treatment of spent nitride fuel, which include preparation of AmCl and electrochemical behavior of Am in LiCl-KCl eutectic melts, are presented.
and electrochemical behavior of Am in LiCl-KCl eutectic melts, are presented.
Hayashi, Hirokazu; Shibata, Hiroki; Akabori, Mitsuo; Arai, Yasuo; Minato, Kazuo
Proceedings of International Conference on Advanced Nuclear Fuel Cycle; Sustainable Options & Industrial Perspectives (Global 2009) (CD-ROM), p.1166 - 1173, 2009/09
R&D on the transmutation of long-lived minor actinides (MA) by the accelerator-driven system (ADS) using nitride fuels is underway at JAEA. In regard to reprocessing technology, pyrochemical process has several advantages in case of treating spent fuel with large decay heat and fast neutron emission, and recovering highly enriched 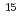 N. In the pyrochemical reprocessing, plutonium and MA are dissolved in LiCl-KCl eutectic melts and selectively recovered into liquid cadmium (Cd) cathode by molten salt electrorefining. The electrochemical behavior in LiCl-KCl eutectic melts and the subsequent nitride formation behavior of plutonium and MA recovered in liquid Cd cathode are investigated. Electrochemical study of americium (Am) on electrolyses of AmN in LiCl-KCl eutectic melts and nitride formation of Am recovered in the liquid Cd cathode are presented. Electrochemical behavior of Am on anodic dissolution of AmN and recovery of Am into a liquid Cd cathode by electrolyses in LiCl-KCl eutectic melts was investigated by transient electrochemical techniques. Am was recovered as Am-Cd alloy in the liquid Cd cathode, in which AmCd
N. In the pyrochemical reprocessing, plutonium and MA are dissolved in LiCl-KCl eutectic melts and selectively recovered into liquid cadmium (Cd) cathode by molten salt electrorefining. The electrochemical behavior in LiCl-KCl eutectic melts and the subsequent nitride formation behavior of plutonium and MA recovered in liquid Cd cathode are investigated. Electrochemical study of americium (Am) on electrolyses of AmN in LiCl-KCl eutectic melts and nitride formation of Am recovered in the liquid Cd cathode are presented. Electrochemical behavior of Am on anodic dissolution of AmN and recovery of Am into a liquid Cd cathode by electrolyses in LiCl-KCl eutectic melts was investigated by transient electrochemical techniques. Am was recovered as Am-Cd alloy in the liquid Cd cathode, in which AmCd type phase was identified besides Cd phase. The recovered Am was converted to AmN by the nitridation-distillation combined method. These results suggest that the pyrochemical process developed for the nitride fuel cycle is applicable for the nitride fuels containing Am, which is to be used for the transmutation of MA.
type phase was identified besides Cd phase. The recovered Am was converted to AmN by the nitridation-distillation combined method. These results suggest that the pyrochemical process developed for the nitride fuel cycle is applicable for the nitride fuels containing Am, which is to be used for the transmutation of MA.
Hayashi, Hirokazu; Shibata, Hiroki; Akabori, Mitsuo; Arai, Yasuo; Minato, Kazuo
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 77(8), p.673 - 676, 2009/08
Times Cited Count:7 Percentile:17.19(Electrochemistry)R&D of the nitride fuel cycle technology is underway at Japan Atomic Energy Agency (JAEA). Behavior of americium (Am) in the pyrochemical process, which includes anodic dissolution of AmN and recovery of Am into a liquid cadmium (Cd) cathode by electrolyses in LiCl-KCl eutectic melts, and nitride formation of Am recovered in liquid Cd, is presented.
Hayashi, Hirokazu; Shibata, Hiroki; Akabori, Mitsuo; Arai, Yasuo; Minato, Kazuo
Proceedings of 2008 Joint Symposium on Molten Salts (USB Flash Drive), p.910 - 915, 2008/10
R&D of the nitride fuel cycle technology is underway at JAEA. Behavior of americium (Am) in the pyrochemical process, which include anodic dissolution of AmN and recovery of Am into liquid Cd cathode by electrorefining in LiCl-KCl eutectic melts, and nitride formation of Am recovered in liquid Cd cathode, are presented.
Hayashi, Hirokazu; Takano, Masahide; Akabori, Mitsuo; Minato, Kazuo
Journal of Alloys and Compounds, 456(1-2), p.243 - 246, 2008/05
Times Cited Count:8 Percentile:45.97(Chemistry, Physical)Americium trichloride was synthesized by the reaction of americium nitride with cadmium chloride at 600-660 K in a dynamic vacuum. The product was hexagonal AmCl , of which lattice parameters were determined to be
, of which lattice parameters were determined to be 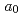 = 0.7390 and
= 0.7390 and 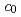 = 0.4215 nm. The results indicate that high purity AmCl
= 0.4215 nm. The results indicate that high purity AmCl samples, in which the oxychloride was not found, were prepared without the use of corrosive reagents. The reaction of the nitrides with cadmium chloride is suitable for synthesis of high purity actinide and lanthanide chlorides.
samples, in which the oxychloride was not found, were prepared without the use of corrosive reagents. The reaction of the nitrides with cadmium chloride is suitable for synthesis of high purity actinide and lanthanide chlorides.
Hayashi, Hirokazu; Akabori, Mitsuo; Minato, Kazuo
Nuclear Technology, 162(2), p.129 - 134, 2008/05
Times Cited Count:13 Percentile:64.63(Nuclear Science & Technology)The electrode reactions of americium at a liquid cadmium electrode were investigated by cyclic voltammetry (CV) of AmCl -(LiCl-KCl)
-(LiCl-KCl)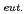 at both 723K and 773K in comparison with those at a molybdenum electrode. The redox peaks assigned to Am(III)/Am(0) (in Cd) were observed with the liquid Cd electrode while the redox reactions of Am(III)/Am(II) and Am(II)/Am(0) were observed with the Mo electrode. The formal standard potential of Am(III)/Am(0) obtained with the liquid Cd electrode is more positive than those calculated for the Mo electrode at both 723K and 773K. The potential shifts were attributed to the lowering of the activity of Am by the formation of the intermetallic compound at the interface between Cd and the molten salt. The Gibbs free energies of formation of the Am-Cd intermetallic compound, which could be AmCd
at both 723K and 773K in comparison with those at a molybdenum electrode. The redox peaks assigned to Am(III)/Am(0) (in Cd) were observed with the liquid Cd electrode while the redox reactions of Am(III)/Am(II) and Am(II)/Am(0) were observed with the Mo electrode. The formal standard potential of Am(III)/Am(0) obtained with the liquid Cd electrode is more positive than those calculated for the Mo electrode at both 723K and 773K. The potential shifts were attributed to the lowering of the activity of Am by the formation of the intermetallic compound at the interface between Cd and the molten salt. The Gibbs free energies of formation of the Am-Cd intermetallic compound, which could be AmCd , are estimated to be -119 and -113 kJ/mol at 723K and 773K, respectively.
, are estimated to be -119 and -113 kJ/mol at 723K and 773K, respectively.
Hayashi, Hirokazu; Akabori, Mitsuo; Minato, Kazuo; Mizuguchi, Koji*; Kawabe, Akihiro*; Fujita, Reiko*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 75(7), p.528 - 534, 2007/07
Times Cited Count:0 Percentile:0.01(Electrochemistry)The simulation code for the pyrochemical processing of spent nuclear fuels was developed to analyze experimental data, to predict experimental results, and to propose adequate conditions and processes. The Simulation code for Pyrochemical Reprocessing (SPR) is based on calculations of chemical equilibrium and electrochemical reactions. The code also includes the calculations of the current-potential distribution between the electrodes. Some calculations were made to simulate the experimental results on the electro-codeposition process of UO and PuO
and PuO . The phenomena of the redox reactions between Pu
. The phenomena of the redox reactions between Pu and Pu
and Pu ions and those between Fe
ions and those between Fe and Fe
and Fe ions were theoretically analyzed; these redox reactions cause the low current efficiency in the electro-codeposition process. The calculated current-potential distribution around the cathode corresponds to the observed distribution of the oxide deposited on the cathode.
ions were theoretically analyzed; these redox reactions cause the low current efficiency in the electro-codeposition process. The calculated current-potential distribution around the cathode corresponds to the observed distribution of the oxide deposited on the cathode.
Shibata, Hiroki; Hayashi, Hirokazu; Minato, Kazuo
JAEA-Research 2007-027, 17 Pages, 2007/03
For application to the preparation of MA chlorides with high-purity, a preparation method, in which corrosive gas like chlorine or hydrogen chloride and so on was not used, of the chloride from the oxide was developed. The preparation conditions of this method were optimized by carrying out preliminary examinations in which gadolinium was used as surrogate for MA. An anhydrous complex chloride ((NH )
) GdCl
GdCl ) was obtained by heating an HCl solution dissolving gadolinium sesquioxide (Gd
) was obtained by heating an HCl solution dissolving gadolinium sesquioxide (Gd O
O ) and ammonium chloride (NH
) and ammonium chloride (NH Cl) under a nitrogen gas flow. An anhydrous GdCl
Cl) under a nitrogen gas flow. An anhydrous GdCl with high-purity was obtained by heating (NH
with high-purity was obtained by heating (NH )
) GdCl
GdCl at 350 degrees under a dynamic vacuum.
at 350 degrees under a dynamic vacuum.
Kuznetsov, S. A.*; Hayashi, Hirokazu; Minato, Kazuo; Gaune-Escard, M.*
Electrochimica Acta, 51(13), p.2463 - 2470, 2006/03
Times Cited Count:90 Percentile:85.75(Electrochemistry)Electrochemical experiments were carried out at 723-823K in order to estimate separation coefficients in LiCl-KCl eutectic melt containing uranium and lanthanum trichlorides. The diffusion coefficients of U(III) were determined by electrochemical methods. The standard rate constants of charge transfer for electroreduction of uranium U(III) +3e =U were calculated by the impedance spectroscopy method. It was shown that for the calculation of uranium and lanthanum separation coefficients it is necessary to determine the voltammetric peak potentials of U(III) and La(III), their concentration in the melt and the kinetic parameters relating to U(III) discharge such as transfer and diffusion coefficients, and standard rate constants of charge transfer.
=U were calculated by the impedance spectroscopy method. It was shown that for the calculation of uranium and lanthanum separation coefficients it is necessary to determine the voltammetric peak potentials of U(III) and La(III), their concentration in the melt and the kinetic parameters relating to U(III) discharge such as transfer and diffusion coefficients, and standard rate constants of charge transfer.
Hayashi, Hirokazu; Akabori, Mitsuo; Minato, Kazuo
Proceedings of International Conference on Nuclear Energy System for Future Generation and Global Sustainability (GLOBAL 2005) (CD-ROM), 3 Pages, 2005/10
For a basis of the future nuclear cycle, it is very important to understand and control the behavior of TRU (Np, Pu, Am, Cm) in the nuclear fuel cycle. Experimental study of pyrochemical process of fuels containing TRU requires the facility having not only shielding for  -ray and neutron but also ability to keep a high purity inert gas atmosphere; because minor actinide chlorides can easily react with oxygen or water vapor in an atmosphere. The module for TRU high temperature chemistry (TRU-HITEC) had been installed to study the basic properties of TRU in the pyrochemical processes. In the present work, the behavior of
-ray and neutron but also ability to keep a high purity inert gas atmosphere; because minor actinide chlorides can easily react with oxygen or water vapor in an atmosphere. The module for TRU high temperature chemistry (TRU-HITEC) had been installed to study the basic properties of TRU in the pyrochemical processes. In the present work, the behavior of  Am in pyrochemical process was investigated by electrochemical methods.
Am in pyrochemical process was investigated by electrochemical methods.
Minato, Kazuo; Akabori, Mitsuo; Tsuboi, Takashi; Kurobane, Shiro; Hayashi, Hirokazu; Takano, Masahide; Otobe, Haruyoshi; Misumi, Masahiro*; Sakamoto, Takuya*; Kato, Isao*; et al.
JAERI-Tech 2005-059, 61 Pages, 2005/09
An experimental facility called the Module for TRU High Temperature Chemistry (TRU-HITEC) was installed in the Back-end Cycle Key Elements Research Facility (BECKY) of the Nuclear Fuel Cycle Safety Engineering Research Facility (NUCEF) for the basic studies of the behavior of the transuranium elements (TRU) in pyrochemical reprocessing and oxide fuels. TRU-HITEC consists of three alpha/gamma cells shielded by steel and polyethylene and a glove box shielded by leaded acrylic resin, where experimental apparatuses have been equipped and a high purity argon gas atmosphere is maintained. In the facility 10 g of  Am as well as the other TRU of Np, Pu and Cm can be handled. This report summarizes the outline, structure, performance and interior apparatuses of the facility, and is the result of the joint research between the Japan Atomic Energy Research Institute and three electric power companies of Tokyo Electric Power Co., Tohoku Electric Power Co. and the Japan Atomic Power Co.
Am as well as the other TRU of Np, Pu and Cm can be handled. This report summarizes the outline, structure, performance and interior apparatuses of the facility, and is the result of the joint research between the Japan Atomic Energy Research Institute and three electric power companies of Tokyo Electric Power Co., Tohoku Electric Power Co. and the Japan Atomic Power Co.
Kuznetsov, S. A.*; Hayashi, Hirokazu; Minato, Kazuo; Gaune-Escard, M.*
Journal of Nuclear Materials, 344(1-3), p.169 - 172, 2005/09
Times Cited Count:26 Percentile:84.04(Materials Science, Multidisciplinary)The knowledge of separation coefficients of actinides and rare-earth metals is important for developing pyrometallurgical process of spent nuclear fuel. Electrochemical experiments were carried out at 723-823 K to estimate separation coefficients in LiCl-KCl eutectic melt containing uranium and lanthanum trichlorides. Uranium and lanthanum separation coefficients is calcurated with the voltammetric peak potentials of U (III) and La (III), their concentration in the melt and kinetic parameters for U(III) discharge such as diffusion coefficients, and standard rate constants of charge transfer. The diffusion coefficients of U (III) were determined by some electrochemical measurements. The standard rate constants of charge transfer for electroreduction of uranium U(III) +3e =U were calculated by impedance spectroscopy method.
=U were calculated by impedance spectroscopy method.