Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Morita, Yasuji; Fukushima, Masahiro; Kashima, Takao*; Tsubata, Yasuhiro
JAEA-Data/Code 2020-013, 38 Pages, 2020/09
Critical Masses of Cm, Am and the mixture were calculated in metal-water mixtures with water reflector as a basic data for criticality safety assessment of minor actinide separation process. In the mixture of Cm-244 and Cm-245, higher ratio of Cm-245 gives smaller critical mass, but the amount of Cm-245 in the critical mass can be obtained by concentration of Cm-245 in the Cm mixture without depending on the Cm-245 ratio. Critical mass of Cm isotope mixture with 30% Cm-245 was smaller than that of Pu isotope mixture in the practical reprocessing (71% Pu-239 + 17% Pu-240 + 12% Pu-241). When Cm is separated from other element including Am and the solution is concentrated, measure for the critical accident has to be taken. Critical mass of Am-242m is smaller than that of Cm-245, but the ratio of Am-242m in the Am contained in practical spent fuel is small enough, about several percent, and therefore the critical accident by Am does not have to be considered. That by the mixture of Am and Cm does not either.
Morita, Yasuji; Tsubata, Yasuhiro
JAEA-Data/Code 2019-015, 45 Pages, 2020/01
Decay heat from radioactive elements in high-level liquid waste (HLLW) and separated solutions in partitioning process was evaluated as a basic data for safety assessment of partitioning process. In the evaluation of HLLW from spent UO fuel burned-up to 45 GWd/t in light water reactor, decay heat value from fission products decreased as the cooling period become longer but heat from actinides, Am and Cm, was almost constant until 50-year cooling. Decay heat density in solutions of Am, Cm and rare earth elements and of Am and Cm without concentration for volume reduction does not exceed the heat density of HLLW, but the concentration should be required to minimize the scale of the partitioning process. Separated solution of Am and Cm must be concentrated to convert the two elements to a solid state to make fuel for transmutation, and the decay heat density of the concentrated solution of Am and Cm is 10 times higher compared with the Pu solution of same element concentration. Higher burn-up UO
fuel burned-up to 45 GWd/t in light water reactor, decay heat value from fission products decreased as the cooling period become longer but heat from actinides, Am and Cm, was almost constant until 50-year cooling. Decay heat density in solutions of Am, Cm and rare earth elements and of Am and Cm without concentration for volume reduction does not exceed the heat density of HLLW, but the concentration should be required to minimize the scale of the partitioning process. Separated solution of Am and Cm must be concentrated to convert the two elements to a solid state to make fuel for transmutation, and the decay heat density of the concentrated solution of Am and Cm is 10 times higher compared with the Pu solution of same element concentration. Higher burn-up UO fuel and MOX fuel in light water reactor and minor-actinide-recycled MOX fuel in fast reactor were also considered and the evaluated decay heat was compared among the spent fuels.
fuel and MOX fuel in light water reactor and minor-actinide-recycled MOX fuel in fast reactor were also considered and the evaluated decay heat was compared among the spent fuels.
Baron, P.*; Cornet, S. M.*; Collins, E. D.*; DeAngelis, G.*; Del Cul, G.*; Fedorov, Y.*; Glatz, J. P.*; Ignatiev, V.*; Inoue, Tadashi*; Khaperskaya, A.*; et al.
Progress in Nuclear Energy, 117, p.103091_1 - 103091_24, 2019/11
Times Cited Count:73 Percentile:94.03(Nuclear Science & Technology)The results of an international review of separation processes for spent nuclear fuel (SNF) recycling in future closed fuel cycles with the evaluation of Technology Readiness Level are reported. This study was made by the Expert Group on Fuel Recycling Chemistry (EGFRC) organised by the Nuclear Energy Agency (NEA) of the Organisation for Economic Co-operation and Development (OECD). A unique feature of this study was that processes were classified according to a hierarchy of separations aimed at different elements within spent fuel (uranium; uranium-plutonium co-recovery; minor actinides; high heat generating radionuclides) and also the Head-end processes, used to prepare the SNF for chemical separation, were included. Separation processes covered both wet (hydrometallurgical) and dry (pyro-chemical) processes.
Morita, Yasuji; Nishihara, Kenji; Tsubata, Yasuhiro
JAEA-Data/Code 2018-017, 32 Pages, 2019/02
Potential radiotoxicity defined as a summation of intake dose was estimated for each actinide element to suppose target of recovery ratio of minor actinide (MA). Importance of each element from the viewpoint of the radiotoxicity was evaluated from the evolution of the radiotoxicity and ratio to the total radiotoxicity. In all the 4 types of spent fuels examined, Am is the most important element. For instance, the potential radiotoxicity of Am accounts for 93% of the total radiotoxicity of actinide elements in HLW produced by reprocessing of spent fuel from pressurized water reactor (PWR). Residual Pu after the recovery of 99.5% in reprocessing still gives contribution that cannot be ignored in radiotoxicity. When the burn-up of the UO fuel in PWR increased, the potential radiotoxicity of actinide elements increased almost in proportion to the burn-up, but in case of MOX fuel in PWR and minor-actinide-recycled MOX fuel in fast reactor, the radiotoxicity of actinide elements increased further. Much consideration is required for the recovery of actinide elements in HLW from different types of fuel.
fuel in PWR increased, the potential radiotoxicity of actinide elements increased almost in proportion to the burn-up, but in case of MOX fuel in PWR and minor-actinide-recycled MOX fuel in fast reactor, the radiotoxicity of actinide elements increased further. Much consideration is required for the recovery of actinide elements in HLW from different types of fuel.
Morita, Yasuji; Yamagishi, Isao
JAEA-Research 2017-006, 27 Pages, 2017/06
Separation of Pd by extraction with 5,8-diethyl-7-hydroxy-6-dodecanone oxime (DEHDO) was examined by batch and continuous tests for the purpose of developing Pd separation process. Batch extraction tests using n-dodecane solution of DEHDO revealed that Pd, Zr and Mo were extracted from simulated high-level radioactive liquid wastes (HLLW) and other elements were not, and also showed that the extraction rate was a little slow and a white precipitate appeared in the aqueous phase but its formation could be avoided by raising temperature. The extracted Pd was found to be back-extracted with sodium nitrite. In the continuous extraction tests with simulated HLLW without Zr and Mo, about 98% of Pd were extracted with DEHDO-n-dodecane and 95% of the extracted Pd were back-extracted with sodium nitrite and nitric acid. Continuous extraction test with simulated HLLW with Zr and Mo showed the possibility of the simultaneous separation of Pd and Mo by DEHDO extraction.
 in a genuine dissolver solution from the results of extraction simulation by PARC-L code
in a genuine dissolver solution from the results of extraction simulation by PARC-L codeAsakura, Toshihide; Hotoku, Shinobu; Morita, Yasuji
Journal of Nuclear Science and Technology, 52(12), p.1552 - 1561, 2015/12
Times Cited Count:1 Percentile:9.74(Nuclear Science & Technology)With a genuine spent fuel soltuion (a dissolver solution), a laboratory-scale reprocessing experiment of an extraction-separation process was performed using mixer-settlers as extactors. In the experiment, n-butyraldehyde was utilized as a reducing reagent of Np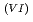 O
O
 to Np
to Np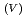 O
O
 for the purpose to distinguish Np
for the purpose to distinguish Np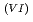 O
O
 from Np
from Np . From the Np concentration in the aqueous phase, Np would be extracted from the dissolver solution together with U and Pu. The scrutiny of Np behavior was performed utilizing 66 cases of calculation results by a Japan Atomic Energy Agency open extraction simulation code, the Programm for Advanced Extraction with Radiation Effect Calculation-Lightened version. From the scrutiny, the authors found that the calculation result with 60% of Np
. From the Np concentration in the aqueous phase, Np would be extracted from the dissolver solution together with U and Pu. The scrutiny of Np behavior was performed utilizing 66 cases of calculation results by a Japan Atomic Energy Agency open extraction simulation code, the Programm for Advanced Extraction with Radiation Effect Calculation-Lightened version. From the scrutiny, the authors found that the calculation result with 60% of Np in the dissolver solution represented the best experimental extraction-separtion behavior of Np. Therefore, it was supposed that the dissolver solution contained sufficient proportion of Np
in the dissolver solution represented the best experimental extraction-separtion behavior of Np. Therefore, it was supposed that the dissolver solution contained sufficient proportion of Np to affect the extraction-separation behavior of Np.
to affect the extraction-separation behavior of Np.
Tsujimoto, Kazufumi; Sasa, Toshinobu; Maekawa, Fujio; Matsumura, Tatsuro; Hayashi, Hirokazu; Kurata, Masaki; Morita, Yasuji; Oigawa, Hiroyuki
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.657 - 663, 2015/09
To continue the utilization of the nuclear fission energy, the management of the high-level radioactive waste is one of the most important issues to be solved. Partitioning and Transmutation technology of HLW is expected to be effective to mitigate the burden of the HLW disposal by reducing the radiological toxicity and heat generation. The Japan Atomic Energy Agency (JAEA) has been conducting the research and development on accelerator-driven subcritical system (ADS) as a dedicated system for the transmutation of long-lived radioactive nuclides. This paper overviews the recent progress and future R&D plan of the study on the ADS and related fuel cycle technology in JAEA.
 -alkylated 2-pyrrolidone derivatives
-alkylated 2-pyrrolidone derivativesTakao, Koichiro*; Kawata, Yoshihisa*; Nogami, Masanobu*; Harada, Masayuki*; Morita, Yasuji; Nishimura, Kenji*; Ikeda, Yasuhisa*
Journal of Nuclear Science and Technology, 52(2), p.294 - 298, 2015/02
Times Cited Count:2 Percentile:17.57(Nuclear Science & Technology)Yields of precipitated UO (NO
(NO )
) (NRP)
(NRP) (NRP =
(NRP =  -alkylated 2-pyrrolidone) were precisely determined by considering reduction of the solution volume through the precipitation, which can be estimated from difference in acid concentrations of the liquid phases before and after the precipitation. The studied NRPs were
-alkylated 2-pyrrolidone) were precisely determined by considering reduction of the solution volume through the precipitation, which can be estimated from difference in acid concentrations of the liquid phases before and after the precipitation. The studied NRPs were  -
- -butyl (NBP) and
-butyl (NBP) and  -
- -propyl (NProP) derivatives. In both systems, the precipitation yields precisely determined were always higher than those simply calculated from the ratio of uranium concentrations before and after the precipitation. However, the differences between them are in the range of 0.6% - 2.6%. If such a difference is practically negligible, the volume reduction through the precipitation does not have to be taken into account for simplicity of the analytical manipulation.
-propyl (NProP) derivatives. In both systems, the precipitation yields precisely determined were always higher than those simply calculated from the ratio of uranium concentrations before and after the precipitation. However, the differences between them are in the range of 0.6% - 2.6%. If such a difference is practically negligible, the volume reduction through the precipitation does not have to be taken into account for simplicity of the analytical manipulation.
Sasaki, Yuji; Kitatsuji, Yoshihiro; Sugo, Yumi; Tsubata, Yasuhiro; Suzuki, Tomoya; Kimura, Takaumi; Morita, Yasuji
Proceedings of 20th International Solvent Extraction Conference (ISEC 2014), p.431 - 435, 2014/09
The eight amide extractants including podand-types, which introduce oxygen, nitrogen and sulfur donor atoms in their central frame, are synthesized and their D values for actinide extraction are compared. The central frame affect greatly on their extractability and it is clear that diglycolamide (DGA) has the highest extractability among extractants used here. In addition, DGA having the different length of alkyl chain or phenyl groups attached to amidic N atoms are obtained and the effect of these substituents is studied. Due to the steric hindrance or hydrogen bond, DGA with long and branched alkyl chains, phenyl group, and proton give relatively low D values.
 -di(2-ethylhexyl)-2,2-dimetnylpropanamide (DEHDMPA) and
-di(2-ethylhexyl)-2,2-dimetnylpropanamide (DEHDMPA) and  -di(2-ethylhexyl)butanamide (DEHBA) using mixer-settler extractors
-di(2-ethylhexyl)butanamide (DEHBA) using mixer-settler extractorsBan, Yasutoshi; Hotoku, Shinobu; Tsubata, Yasuhiro; Morita, Yasuji
Solvent Extraction and Ion Exchange, 32(4), p.348 - 364, 2014/05
Times Cited Count:9 Percentile:31.46(Chemistry, Multidisciplinary)Extraction properties of  -di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) for nitric acid, U(VI), and Pu(IV) were studied, and the distribution ratio equations were derived for each chemical species. A continuous counter-current experiment was performed using mixer-settler extractors with two types of monoamides, DEHDMPA and
-di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) for nitric acid, U(VI), and Pu(IV) were studied, and the distribution ratio equations were derived for each chemical species. A continuous counter-current experiment was performed using mixer-settler extractors with two types of monoamides, DEHDMPA and  -di(2-ethylhexyl)butanamide (DEHBA), as extractants. DEHDMPA exclusively extracted U from the feed, and the ratio of U recovered in the U fraction stream was 99.93%. Almost all Pu were extracted by DEHBA, and the recovery of Pu in the U-Pu fraction stream was 99.94%. Concentrations of U and Pu in mixer-settlers were calculated using a simulation code, which confirmed that the calculation was effective for estimating the U concentration in the U fraction stream, and the U and Pu concentrations in the U-Pu fraction stream.
-di(2-ethylhexyl)butanamide (DEHBA), as extractants. DEHDMPA exclusively extracted U from the feed, and the ratio of U recovered in the U fraction stream was 99.93%. Almost all Pu were extracted by DEHBA, and the recovery of Pu in the U-Pu fraction stream was 99.94%. Concentrations of U and Pu in mixer-settlers were calculated using a simulation code, which confirmed that the calculation was effective for estimating the U concentration in the U fraction stream, and the U and Pu concentrations in the U-Pu fraction stream.
Sasaki, Yuji; Tsubata, Yasuhiro; Kitatsuji, Yoshihiro; Sugo, Yumi; Shirasu, Noriko; Morita, Yasuji
Proceedings of International Nuclear Fuel Cycle Conference; Nuclear Energy at a Crossroads (GLOBAL 2013) (CD-ROM), p.1079 - 1082, 2013/09
Mutual separation of Am, Cm and lanthanides (Ln) is important to develop the partitioning process of high-level radioactive liquid waste, because the application to their different disposal methods are advantageous. Namely, Am is studied for transmutation due to the reduction of long half-life radionuclides, Cm should be kept in interim storage in order to reduce the calorific value, and Ln should be present in the vitrified radioactive waste toward the geological disposal. However, this mutual separation method is difficult to establish because they have very similar chemical behavior, same oxidation state (III) and similar ionic radii. The development of their mutual separation is termed as the challenging study. In order to obtain the satisfactory results, the property of extractant requires the differentiation of actinide (An) from Ln, high preferability to different ionic radii between Am and Cm, and high extractability to hard acids. Therefore, the extractant have to include both N atom, whose soft donor has high selectivity between An and Ln, and O atoms for the strong extractability to An. The new extractant, NTAamide (N,N,N',N',N'',N''-hexaoctyl-nitrirotriacetamide) is a triamide having N donor at the center of backbone, then NTAamide has hybrid performance of complexation to metals by soft N and three hard amidic O atoms. It is clear that NTAamide can extract trivalent An at diluted HNO with small D(Ln), the separation of An from Ln can be carried out at that condition. The SF of Am/Cm by NTAamide is approximate 1.8, which is not so high to separate each other. The combination of NTAamide of extractant and TEDGA (N,N,N',N'-tetraethyl-diglycolamide) as a masking agent in the aqueous phase shows very high SF(Am/Cm) of maximal 6.5. It is obvious that NTAamide is a promising extractant to achieve the mutual separation among Am/Cm/Ln.
with small D(Ln), the separation of An from Ln can be carried out at that condition. The SF of Am/Cm by NTAamide is approximate 1.8, which is not so high to separate each other. The combination of NTAamide of extractant and TEDGA (N,N,N',N'-tetraethyl-diglycolamide) as a masking agent in the aqueous phase shows very high SF(Am/Cm) of maximal 6.5. It is obvious that NTAamide is a promising extractant to achieve the mutual separation among Am/Cm/Ln.
 -di(2-ethylhexyl)butanamide (DEHBA) in mixer-settler extractors
-di(2-ethylhexyl)butanamide (DEHBA) in mixer-settler extractorsBan, Yasutoshi; Hotoku, Shinobu; Tsubata, Yasuhiro; Morita, Yasuji
Solvent Extraction and Ion Exchange, 31(6), p.590 - 603, 2013/09
Times Cited Count:10 Percentile:37.86(Chemistry, Multidisciplinary)The recovery of U and Pu from nitric acid using the monoamide extractant  -di(2-ethylhexyl)butanamide (DEHBA) in mixer-settler extractors was calculated using a simulation code, and a continuous counter-current experiment using mixer-settler extractors was performed. The flow rate, stage number, and nitric acid concentration were chosen as the parameters for the calculation, and the appropriate experimental conditions for separating U from Pu were determined. The results of the continuous counter-current experiment showed that the percentages of U and Pu extracted using 1.5 mol/dm
-di(2-ethylhexyl)butanamide (DEHBA) in mixer-settler extractors was calculated using a simulation code, and a continuous counter-current experiment using mixer-settler extractors was performed. The flow rate, stage number, and nitric acid concentration were chosen as the parameters for the calculation, and the appropriate experimental conditions for separating U from Pu were determined. The results of the continuous counter-current experiment showed that the percentages of U and Pu extracted using 1.5 mol/dm (M) DEHBA from 4 M nitric acid were
(M) DEHBA from 4 M nitric acid were  99.9% and 97.84%, respectively.
99.9% and 97.84%, respectively.
Hayashi, Hirokazu; Hagiya, Hiromichi; Kim, S.-Y.*; Morita, Yasuji; Akabori, Mitsuo; Minato, Kazuo
Journal of Radioanalytical and Nuclear Chemistry, 296(3), p.1275 - 1286, 2013/06
Times Cited Count:4 Percentile:32.48(Chemistry, Analytical) Cm was separated and recovered as an oxalate from
Cm was separated and recovered as an oxalate from  Cm-
Cm- Pu mixed oxide which had been
Pu mixed oxide which had been  Cm oxide sample prepared 40 years ago. Plutonium ions were removed from the solution prepared by dissolution of
Cm oxide sample prepared 40 years ago. Plutonium ions were removed from the solution prepared by dissolution of  Cm-
Cm- Pu mixed oxide in nitric acid, by using an anion exchange resin column. Curium oxalate, a precursor compound of curium oxide, was prepared from the purified curium solution and supplied for the syntheses and measurements of the thermochemical properties of curium compounds.
Pu mixed oxide in nitric acid, by using an anion exchange resin column. Curium oxalate, a precursor compound of curium oxide, was prepared from the purified curium solution and supplied for the syntheses and measurements of the thermochemical properties of curium compounds.
 -ray irradiation in HNO
-ray irradiation in HNO media
mediaNogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 296(1), p.423 - 427, 2013/04
Times Cited Count:5 Percentile:38.62(Chemistry, Analytical)Stability of polyvinylpolypyrrolidone (PVPP), a resin with adsorption selectivity to U(VI) in nitric acid media, against  -ray irradiation has been examined using HNO
-ray irradiation has been examined using HNO solutions of various concentrations. As the result, no clear decrease in the capacity was observed for any samples. Or rather, it was found that the capacity increased by approximately 50% for the PVPP slurry irradiated in 6 M HNO
solutions of various concentrations. As the result, no clear decrease in the capacity was observed for any samples. Or rather, it was found that the capacity increased by approximately 50% for the PVPP slurry irradiated in 6 M HNO . The infrared spectroscopic study indicates that PVPP degrades by
. The infrared spectroscopic study indicates that PVPP degrades by  -ray irradiation in HNO
-ray irradiation in HNO from the cleavage of the pyrrolidone ring by the addition of oxygen atom originating from HNO
from the cleavage of the pyrrolidone ring by the addition of oxygen atom originating from HNO , followed by the formation of chain monoamides with multiple coordinative atoms by the continuous addition of oxygen, finally leading to the generation of primary-amine type anion exchange resin. It is also indicated that all generated functional groups possess adsorptivity to U(VI) in 3 M HNO
, followed by the formation of chain monoamides with multiple coordinative atoms by the continuous addition of oxygen, finally leading to the generation of primary-amine type anion exchange resin. It is also indicated that all generated functional groups possess adsorptivity to U(VI) in 3 M HNO .
.
Fujii, Toshiyuki*; Egusa, Soichiro*; Uehara, Akihiro*; Yamana, Hajimu*; Morita, Yasuji
Journal of Radioanalytical and Nuclear Chemistry, 295(3), p.2059 - 2062, 2013/03
Times Cited Count:3 Percentile:18.63(Chemistry, Analytical)Quantitative analysis of Nd, U, or Pd in 3 mol dm (M) HNO
(M) HNO was performed by reflection absorption spectrophotometry at ultraviolet-visible-near-infrared (UV/Vis/NIR) region. A sample chamber with optics for reflection measurement was designed and attached to a UV/Vis/NIR spectrophotometer by optical fibers. The reflection absorbance showed linear relations with concentrations of Nd, U, and Pd at the absorbance region less than 0.1. The quantitative analysis was found to be possible for 3 M HNO
was performed by reflection absorption spectrophotometry at ultraviolet-visible-near-infrared (UV/Vis/NIR) region. A sample chamber with optics for reflection measurement was designed and attached to a UV/Vis/NIR spectrophotometer by optical fibers. The reflection absorbance showed linear relations with concentrations of Nd, U, and Pd at the absorbance region less than 0.1. The quantitative analysis was found to be possible for 3 M HNO solutions containing [Nd] ca. 0.2 M, [U] ca. 0.04 M, or [Pd] ca. 0.01 M at maximum.
solutions containing [Nd] ca. 0.2 M, [U] ca. 0.04 M, or [Pd] ca. 0.01 M at maximum.
Sasaki, Yuji; Tsubata, Yasuhiro; Kitatsuji, Yoshihiro; Morita, Yasuji
Chemistry Letters, 42(1), p.91 - 92, 2013/01
Times Cited Count:41 Percentile:72.41(Chemistry, Multidisciplinary)The new N-donor extractant, NTAamide, is synthesized and tested for extraction and separation of Am-Cm-Ln. It was found that the high separation factors for An-Ln can be seen at 0.1-0.2M HNO , which suggest the de-protonation to N donor is important to show the high SF between An and Ln. From the present work, the stable N-donor extractant having high SF of An-Ln from nitric acid to n-dodecane can be obtained. The highest separation factor, 6.5, of Am-Cm for the condition of 0.5M NTAamide (C8) in n-dodecane and 10 mM TEDGA in 0.2M HNO
, which suggest the de-protonation to N donor is important to show the high SF between An and Ln. From the present work, the stable N-donor extractant having high SF of An-Ln from nitric acid to n-dodecane can be obtained. The highest separation factor, 6.5, of Am-Cm for the condition of 0.5M NTAamide (C8) in n-dodecane and 10 mM TEDGA in 0.2M HNO as one of the highest SF values is found. NTAamide is the promising extractants for partitioning process of Am-Cm-Ln.
as one of the highest SF values is found. NTAamide is the promising extractants for partitioning process of Am-Cm-Ln.
Sugo, Yumi; Taguchi, Mitsumasa; Sasaki, Yuji; Morita, Yasuji; Ishioka, Noriko
JAEA-Review 2012-046, JAEA Takasaki Annual Report 2011, P. 18, 2013/01
 nitric acid at 373 K
nitric acid at 373 KBan, Yasutoshi; Morita, Yasuji
Radiochimica Acta, 100(12), p.879 - 883, 2012/12
Times Cited Count:3 Percentile:24.98(Chemistry, Inorganic & Nuclear)Optical absorption spectra of Pu(IV) in 3 mol/dm (M) nitric acid at 373 K were measured using an optical quartz cell equipped with a thin-film heater. Pu(IV) was oxidized to Pu(VI) by heating at 373 K, and the concentrations of Pu(IV) and Pu(VI) gradually decreased and increased, respectively, with the heating time. The ratio of Pu(IV) oxidized to Pu(VI) was 37% at a heating time of 394 min, and no Pu(III) or Pu(V) characteristic peaks were observed throughout the experiment. The oxidation rate of Pu(IV) in 3 M nitric acid at 373 K could be described by the equation -d[Pu(IV)]
(M) nitric acid at 373 K were measured using an optical quartz cell equipped with a thin-film heater. Pu(IV) was oxidized to Pu(VI) by heating at 373 K, and the concentrations of Pu(IV) and Pu(VI) gradually decreased and increased, respectively, with the heating time. The ratio of Pu(IV) oxidized to Pu(VI) was 37% at a heating time of 394 min, and no Pu(III) or Pu(V) characteristic peaks were observed throughout the experiment. The oxidation rate of Pu(IV) in 3 M nitric acid at 373 K could be described by the equation -d[Pu(IV)] /d
/d =
=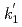 [Pu(IV)]
[Pu(IV)] -
-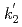 [Pu(VI)]
[Pu(VI)] , where [Pu(IV)]
, where [Pu(IV)] and [Pu(VI)]
and [Pu(VI)] were the concentrations of Pu(IV) and Pu(VI) at a heating time of t min. The apparent rate constants for Pu(IV) oxidation (
were the concentrations of Pu(IV) and Pu(VI) at a heating time of t min. The apparent rate constants for Pu(IV) oxidation (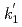 ) and Pu(VI) reduction (
) and Pu(VI) reduction (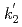 ) at 373 K were calculated to be 2.3
) at 373 K were calculated to be 2.3 10
10 min
min and 3.4
and 3.4 10
10 min
min , respectively.
, respectively.
Morita, Yasuji; Yamagishi, Isao; Tsubata, Yasuhiro; Matsumura, Kazumi; Sakurai, Koji*; Iijima, Takahiko
JAEA-Research 2012-031, 39 Pages, 2012/11
Solvent extraction process with di-2-ethylhexylphosphoric acid has been developed for the purpose of Mo separation from high-level radioactive liquid wastes (HLLW). Mo has very low solubility in borosilicate glasses and makes so-called yellow phase when it contained beyond the solubility. After extraction and back-extraction data of Mo and other fission products were obtained by batch extraction tests, continuous extraction tests with simulated HLLW were performed using mixer-settler twice. At the second test, reduction of Y extraction yield and increase of Mo and Zr back-extraction yield were obtained compared with the results of the first tests, but those values should be still improved. Process simulation technique was developed using simulation code named PARC-MA, and the optimized process condition was obtained by the simulation.
 -ray irradiation
-ray irradiationNogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Science China; Chemistry, 55(9), p.1739 - 1745, 2012/09
Times Cited Count:4 Percentile:19.69(Chemistry, Multidisciplinary)Stability of N-alkylated pyrrolidone derivatives (NRPs) against radiation was examined by irradiation tests with  Co
Co  -ray. We have been developed a novel reprocessing system using NRPs which have precipitation ability to haxa- and tetravalent actinides in nitric acid media. Degradation rates of NRPs are evaluated by irradiation in 3M nitric acid solutions and mechanism of degradation are discussed in the present paper.
-ray. We have been developed a novel reprocessing system using NRPs which have precipitation ability to haxa- and tetravalent actinides in nitric acid media. Degradation rates of NRPs are evaluated by irradiation in 3M nitric acid solutions and mechanism of degradation are discussed in the present paper.