Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sasaki, Takayuki*; Yoshida, Hatsumi*; Kitatsuji, Yoshihiro; Takagi, Ikuji*; Moriyama, Hirotake*
Chemistry Letters, 40(8), p.870 - 871, 2011/08
Times Cited Count:1 Percentile:6.74(Chemistry, Multidisciplinary)Trivalent metal ion selective electrode (ISE) consisting of bis-(diphenylphosphoryl)-methane as an ionophore was developed for the determination of formation constants with organic ligands. The ISE prepared exhibited Nernstian response to the concentration of Eu in the test solutions. Even in the presence of carboxylic acids such as malonic acid in samples, the obtained potential values were found to be stable and reproducible during the measurement time. The present formation constants of Eu with carboxylates were in good agreement with the reported values.
in the test solutions. Even in the presence of carboxylic acids such as malonic acid in samples, the obtained potential values were found to be stable and reproducible during the measurement time. The present formation constants of Eu with carboxylates were in good agreement with the reported values.
Yamaguchi, Akira*; Yanagisawa, Tsutomu; Moriyama, Hirotake*
Nihon Genshiryoku Gakkai-Shi ATOMO , 52(10), p.626 - 637, 2010/10
, 52(10), p.626 - 637, 2010/10
This article introduces the innovative technologies that examine the adoption to the sodium-cooled fast reactor as an approach for commercial use of the fast reactor cycle system, as the final of serial, mainly by International Conference (FR09) organized by IAEA and held in December, 2009. The latest situation of the research and development and the simulation technology concerning a reactor technologies such as the reactor configuration, the reactor system design, and the cooling system devices are reviewed. Fuel development, wet reprocessing and pyro-reprocessing technologies are also reviewed by the views for the future. In addition, the problem of safety, a nuclear nonproliferation, an operating experience, a technological transfer between generation, and the human resource is described, including the expectation to Japan by the utilization of Monju and Joyo.
Toda, Taro*; Maruyama, Takehiro*; Moritani, Kimikazu*; Moriyama, Hirotake*; Hayashi, Hirokazu
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 77(8), p.649 - 651, 2009/08
Times Cited Count:8 Percentile:19.4(Electrochemistry)The distribution coefficients of Am and Ce were measured in the eutectic LiCl-KCl/liquid Ga system at 773K. By using ZrCl as the oxide ion scavenger in order to avoid the formation of such oxychlorides as MO
as the oxide ion scavenger in order to avoid the formation of such oxychlorides as MO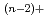 , the effect of oxide ion concentration was well controlled on the distribution coefficients of Am and Ce. The separation factor between Am and Ce was then obtained to be about 100. By comparing the present value with the other experimental and the predicted ones, it was confirmed that the Ga system was more selective than the Bi and Cd system.
, the effect of oxide ion concentration was well controlled on the distribution coefficients of Am and Ce. The separation factor between Am and Ce was then obtained to be about 100. By comparing the present value with the other experimental and the predicted ones, it was confirmed that the Ga system was more selective than the Bi and Cd system.
Moriyama, Hirotake*; Moritani, Kimikazu*; Toda, Taro*; Hayashi, Hirokazu
Radiochimica Acta, 97(4-5), p.233 - 236, 2009/05
Times Cited Count:1 Percentile:10.22(Chemistry, Inorganic & Nuclear)The distribution coefficients of Am, Ce, and Eu between the salt and metal phases were measured at 1073 K in a reductive extraction system of equimolar NaCl-KCl melt and liquid Ga, which were expected to be higher from a thermodynamic point of view. By changing some solute concentrations, it was observed that the distribution coefficients were much dependent on the oxide ion concentration in the system, possibly due to the formation of such compounds as AmO and CeO
and CeO . Also, in some runs, the mass balance of Am was affected possibly by the formation and precipitation of its oxychloride and/or oxide. By taking the observations into consideration, the separation factor between Am and Ce was obtained to be about 30 as a lower limit in the present system.
. Also, in some runs, the mass balance of Am was affected possibly by the formation and precipitation of its oxychloride and/or oxide. By taking the observations into consideration, the separation factor between Am and Ce was obtained to be about 30 as a lower limit in the present system.
Takagi, Ikuji*; Kobayashi, Takashi*; Ueyama, Yutaka*; Moriyama, Hirotake*; Nakamichi, Masaru; Nakamura, Hirofumi; Hayashi, Kimio
Journal of Nuclear Materials, 386-388, p.682 - 684, 2009/04
Times Cited Count:8 Percentile:49.52(Materials Science, Multidisciplinary)no abstracts in English
Toda, Taro*; Maruyama, Takehiro*; Moritani, Kimikazu*; Moriyama, Hirotake*; Hayashi, Hirokazu
Journal of Nuclear Science and Technology, 46(1), p.18 - 25, 2009/01
Times Cited Count:41 Percentile:92.56(Nuclear Science & Technology)The excess thermodynamic quantities of lanthanides and actinides in molten salts and liquid metals were studied for reductive extraction of minor actinides. The excess enthalpies and entropies of those elements in the molten chloride phase were found to be correlated with the ionic radii of metal ions possibly due to complex formation. In the liquid metal phase, on the other hand, the excess enthalpies were explained with Miedema's atomistic model and the excess entropies were explained with the vibrational entropy due to alloy formation. Using these correlations and models, some missing values of the excess thermodynamic quantities were evaluated and the separation factors of minor actinides from lanthanides were calculated in different reductive extraction systems. The higher separation factors were obtained in the system using aluminum or gallium than in the system using bismuth or cadmium as the liquid metal phase.
Toda, Taro*; Maruyama, Takehiro*; Moritani, Kimikazu*; Moriyama, Hirotake*; Hayashi, Hirokazu
Proceedings of 2008 Joint Symposium on Molten Salts (USB Flash Drive), p.933 - 938, 2008/10
The distribution coefficients of Am and Ce were measured in the LiCl-KCl/Ga system at 773 K. By using ZrCl as the oxide ion scavenger in order to avoid the formation of such oxychlorides as CeO
as the oxide ion scavenger in order to avoid the formation of such oxychlorides as CeO and AmO
and AmO , the effect of oxide ion concentration was well controlled on the distribution coefficients of Am and Ce. The separation factor between Am and Ce was then obtained to be about 100. By comparing the present value with the other experimental and the predicted ones, it was confirmed that solvent metals were ordered from the most selective to the less selective one as Al
, the effect of oxide ion concentration was well controlled on the distribution coefficients of Am and Ce. The separation factor between Am and Ce was then obtained to be about 100. By comparing the present value with the other experimental and the predicted ones, it was confirmed that solvent metals were ordered from the most selective to the less selective one as Al Ga
Ga Bi
Bi Cd.
Cd.
Sasaki, Takayuki*; Kubo, Shintaro*; Kobayashi, Taishi*; Kirishima, Akira*; Kimura, Takaumi; Kubota, Takumi*; Takagi, Ikuji*; Moriyama, Hirotake*
Journal of Nuclear and Radiochemical Sciences, 6(1), p.51 - 54, 2005/07
no abstracts in English
 , PuO
, PuO and rare earth oxides using ZrCl
and rare earth oxides using ZrCl in LiCl-KCl eutectic melt
in LiCl-KCl eutectic meltSakamura, Yoshiharu*; Inoue, Tadashi*; Iwai, Takashi; Moriyama, Hirotake*
Journal of Nuclear Materials, 340(1), p.39 - 51, 2005/04
Times Cited Count:40 Percentile:91.4(Materials Science, Multidisciplinary)A new chlorination method using ZrCl in a molten salt has been investigated for the pyrometallurgical reprocessing of spent oxide fuels. UO
in a molten salt has been investigated for the pyrometallurgical reprocessing of spent oxide fuels. UO , PuO
, PuO and rare earth oxides(La
and rare earth oxides(La O
O , CeO
, CeO , Nd
, Nd O
O and Y
and Y O
O ) were allowed to react with ZrCl
) were allowed to react with ZrCl in a LiCl-KCl eutectic salt at 500
in a LiCl-KCl eutectic salt at 500 C to give a metal chloride solution and a precipitate of ZrO
C to give a metal chloride solution and a precipitate of ZrO . By keeping the system quite still, the solution settled so that the ZrO
. By keeping the system quite still, the solution settled so that the ZrO precipitate could be separated.
precipitate could be separated.
Moriyama, Hirotake*
JNC TJ8400 2004-029, 45 Pages, 2005/02
In support of the safety assessment of geologic disposal of high level radioactive wastes, solubility measurement of Zr(IV) was performed in a pH range from 1 to 14 at ionic strength of 0.1, 0.5 and 1.0M. The values of solubility product and hydrolysis constant were obtained from the solubility data. Also, the polynuclear hydrolysis constants reported for hexavalent actinide ions were examined by using a hard sphere model, and some predictions were made.
Moriyama, Hirotake*
JNC TJ8400 2003-058, 41 Pages, 2004/02
In support of the safety assessment of geologic disposal of high level radioactive wastes, solubility measurement of U(IV) in alkaline solution was performed. The solubility of U(IV) was observed to increase in alkaline solution, and the hydrolysis constants beta
 and beta
and beta
 were obtained. Reported thermodynamic data of solubility products, hydrolysis constants and complexation constants were also compared and discussed. It was found that uncertainty of the reported beta
were obtained. Reported thermodynamic data of solubility products, hydrolysis constants and complexation constants were also compared and discussed. It was found that uncertainty of the reported beta
 values of tetravalent actinide ions is still large. The beta
values of tetravalent actinide ions is still large. The beta
 values were estimated by using a hard sphere model extended to 8 coordination, showing that such large uncertainty is due to the effect of colloid formation.
values were estimated by using a hard sphere model extended to 8 coordination, showing that such large uncertainty is due to the effect of colloid formation.
Takebe, Shinichi; Fujiwara, Keiji*; Moriyama, Hirotake*
Nihon Genshiryoku Gakkai "Shisetsu, Kankyo Hoshano Dotai" Kenkyu Senmon Iinkai Hokokusho, p.1 - 9, 2003/03
no abstracts in English
Moriyama, Hirotake*
JNC TJ8400 2002-054, 34 Pages, 2003/02
In support of the safety assessment of geologic disposal of high level radioactive wastes, hydrolysis reactions of U(IV) were studied. The hydrolysis constants of tetravalent uranium were determined by solvent extraction method using thenoyltrifluoroacetone(TTA) and  U. The distribution ratio of U(IV) was measured as a function of the pH
U. The distribution ratio of U(IV) was measured as a function of the pH value in the aqueous phase at I=0.1, 0.5 and 1.0, and was analyzed to obtain the hydrolysis constants of
value in the aqueous phase at I=0.1, 0.5 and 1.0, and was analyzed to obtain the hydrolysis constants of 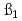 ,
, 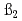 and
and 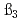 at the standard state (I=0). The higher order hydrolysis constants of
at the standard state (I=0). The higher order hydrolysis constants of 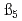 and
and  were also obtained from the results of the solubility measurement at a high pH condition. The systematic behavior of the hydrolysis constants was by using an improved hard sphere model.
were also obtained from the results of the solubility measurement at a high pH condition. The systematic behavior of the hydrolysis constants was by using an improved hard sphere model.
*; Fujii, Toshiyuki*; Moriyama, Hirotake*; ; ; Myochin, Munetaka; Ojima, Hisao
JNC TY8400 2002-013, 57 Pages, 2002/03
no abstracts in English
Yamana, Hajimu*; Moriyama, Hirotake*; Asano, Hideki*; Shiokawa, Yoshinobu*; Yamamura, Tomoo*; Hasegawa, Kazuki*; Kimura, Akihiro*; Umekita, Satoshi*
JAERI-Tech 2002-018, 33 Pages, 2002/03
no abstracts in English
Moriyama, Hirotake*
JNC TJ8400 2001-048, 40 Pages, 2002/02
In support of the safety assessment of geologic disposal of high level radioactive wastes, hydrolysis and complexation reactions of transuranium elements were studied. For comparison with the case of Pu(IV), the solubility of UO .xH
.xH O was measured under a reducing condition, and the solubility product K
O was measured under a reducing condition, and the solubility product K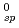 and some hydrolysis constants were obtained. Similarly to the value of Pu(IV), the obtained K
and some hydrolysis constants were obtained. Similarly to the value of Pu(IV), the obtained K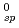 value was found to be considerably smaller than that predicted by Rai et al. from its dependence on ionic radius. Also, the solubility of UO
value was found to be considerably smaller than that predicted by Rai et al. from its dependence on ionic radius. Also, the solubility of UO (OH)
(OH) under an oxidizing condition was measured, and the solubility product K
under an oxidizing condition was measured, and the solubility product K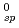 was obtained. For complexation, furthermore, it was examined by using an improved hard sphere model to predict some complexation constants of actinide ions which were lacked in literatures.
was obtained. For complexation, furthermore, it was examined by using an improved hard sphere model to predict some complexation constants of actinide ions which were lacked in literatures.
Moriyama, Hirotake*
JNC TJ8400 2001-015, 48 Pages, 2001/03
In suport of the safety assessment of geologic disposal of high level radioactivc wastes, the solubility of transuranium elements was studied. The ionic strength dependence of solubility of PuO
 xH
xH O was measured under a reducing condition, and the solubility product K
O was measured under a reducing condition, and the solubility product K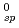 was obtained. The obtained K
was obtained. The obtained K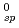 value was found to be much smaller than that predicted by Rai et al. from its dependence on ionic radius. Also, the systematic trends of complex formation constants of actinide ions were cxamined by using an improved hard sphere model. It was found that the systematic trends of the literature values for actinide ions other than An
value was found to be much smaller than that predicted by Rai et al. from its dependence on ionic radius. Also, the systematic trends of complex formation constants of actinide ions were cxamined by using an improved hard sphere model. It was found that the systematic trends of the literature values for actinide ions other than An were well explained by the present model. The reliability of the literature values for An
were well explained by the present model. The reliability of the literature values for An would be checked.
would be checked.
Moriyama, Hirotake*
JNC TJ8400 2000-050, 47 Pages, 2000/03
In support of the safety assessment of geologic disposal of high levcl radioactive wastes, the solubility of transuranium elements was studied. The solubility of PuO
 xH
xH O was measured undcr a reducing condition, and the solubility product K
O was measured undcr a reducing condition, and the solubility product K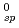 and the stability constant
and the stability constant 
 of Pu(OH)
of Pu(OH) were obtained. The obtained K
were obtained. The obtained K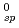 value was found to be much smaller than that predicted by Rai et al. from its dependence on ionic radius. Also, the solubility of PuO
value was found to be much smaller than that predicted by Rai et al. from its dependence on ionic radius. Also, the solubility of PuO 3
3  xH
xH O was measured under an oxidizing condition and the solubility product K
O was measured under an oxidizing condition and the solubility product K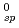 was obtained. In the analysis of hydrolysis constants of actinide ions, it was found that the systematic trend of the hydrolysis constants was well explained by the hard sphere model considering the effective charges of actinide ions.
was obtained. In the analysis of hydrolysis constants of actinide ions, it was found that the systematic trend of the hydrolysis constants was well explained by the hard sphere model considering the effective charges of actinide ions.
Moriyama, Hirotake*
JNC TJ8400 99-060, 26 Pages, 1999/02
In support of the safety assessment of geologic disposal of high level radioactive wastes, the solubility of transuranium elements was studied. The solubility of plutonium was measured in an aqueous solution of pH3-8 under reducing conditions, and the obtained results were well explained by considering the presence of the solution species of Pu(III) in equilibrium with the solid phase of PuO (am). Also, systematic behavior of hydrolysis constants of actinide ions was studied with the help of a simple hard sphere model. The literature data of the hydrolysis constants of actinide ions were analyzed by the model and systematic trends were explained by taking into account the effective charges of actinide ions. Such a model is useful to check the experimental data and to predict the unknown data from a systematic point of view.
(am). Also, systematic behavior of hydrolysis constants of actinide ions was studied with the help of a simple hard sphere model. The literature data of the hydrolysis constants of actinide ions were analyzed by the model and systematic trends were explained by taking into account the effective charges of actinide ions. Such a model is useful to check the experimental data and to predict the unknown data from a systematic point of view.
Moriyama, Hirotake*
PNC TJ1604 92-003, 39 Pages, 1992/03
no abstracts in English