Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...

 and
and 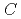
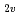 isomers of C
isomers of C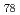
Han, A. H.*; Wakahara, Takatsugu*; Maeda, Yutaka*; Akasaka, Takeshi*; Fujitsuka, Mamoru*; Ito, Osamu*; Yamamoto, Kazunori; Kako, Masahiro*; Kobayashi, Kaoru*; Nagase, Shigeru*
New Journal of Chemistry, 33(3), p.497 - 500, 2009/03
Times Cited Count:7 Percentile:32.52(Chemistry, Multidisciplinary)A new chemical method can be applied to isolate the isomers of C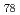 . The photochemical cycloaddition of a mixture of C
. The photochemical cycloaddition of a mixture of C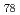 (
(
 ) and C
) and C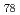 (
(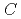
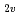 ) with disilirane affords only the mono-adduct of the
) with disilirane affords only the mono-adduct of the 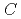
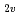 isomer of C
isomer of C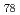 . The counter part (C
. The counter part (C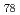 (
(
 )) does not give the mono-adduct with disilirane. The
)) does not give the mono-adduct with disilirane. The 
 isomer itself and the adduct of the
isomer itself and the adduct of the 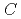
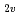 isomer of C
isomer of C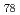 with disilirane was easily separated by a HPLC procedure. A facile oxidative desilylation of the adduct took place, resulting in the formation of pristine C
with disilirane was easily separated by a HPLC procedure. A facile oxidative desilylation of the adduct took place, resulting in the formation of pristine C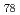 (
(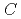
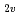 ). In this context, the separation and the isolation of two isomers of C
). In this context, the separation and the isolation of two isomers of C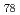 (
(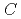
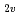 ) and C
) and C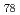 (
(
 ) were successfully accomplished by using a silylation-oxidative desilylation process.
) were successfully accomplished by using a silylation-oxidative desilylation process.
 have an anomalous endohedral structure? Synthesis and single crystal X-ray structure of the carbene adduct
have an anomalous endohedral structure? Synthesis and single crystal X-ray structure of the carbene adductAkasaka, Takeshi*; Kono, Takayoshi*; Takematsu, Yuji*; Nikawa, Hidefumi*; Nakahodo, Tsukasa*; Wakahara, Takatsugu*; Ishitsuka, Midori*; Tsuchiya, Takahiro*; Maeda, Yutaka*; Liu, M. T. H.*; et al.
Journal of the American Chemical Society, 130(39), p.12840 - 12841, 2008/10
Times Cited Count:76 Percentile:84.42(Chemistry, Multidisciplinary)We report here the results on single crystal X-ray crystallographic analysis of the Gd@C carbene adduct (Gd@C
carbene adduct (Gd@C (ad), Ad=adamantylidene). The Gd atom in Gd@C
(ad), Ad=adamantylidene). The Gd atom in Gd@C (Ad) is located at an off-centered position near a hexagonal ring in the C2v-C
(Ad) is located at an off-centered position near a hexagonal ring in the C2v-C cage, as found for M@C
cage, as found for M@C (M = Sc and La) and La@C
(M = Sc and La) and La@C (Ad). Theoretical calculation also confirms the position of the Gd atom in the X-ray crystal structure.
(Ad). Theoretical calculation also confirms the position of the Gd atom in the X-ray crystal structure.

Akasaka, Takeshi*; Kato, Tatsuhisa*; Kobayashi, Kaoru*; Nagase, Shigeru*; Yamamoto, Kazunori; Funasaka, Hideyuki; Takahashi, Takeshi
Nature, 374, p.600 - 601, 1994/04
Times Cited Count:156 Percentile:99.11(Multidisciplinary Sciences)no abstracts in English
Nagase, Midori*; Arai, Tsuyoshi*; Sano, Yuichi; Watanabe, So; Kunii, Shigeru*
no journal, ,
As a separation technology for recovering MA (III) from high-level radioactive liquid waste, we focused on an adsorbent impregnated with an NTA amide extractant. In this study, we synthesized H2EH NTA extractant, which is one of the NTA amide extractants, and investigated the adsorption / elution behavior for rare earth elements.
Yoshida, Masaaki*; Nemoto, Shuhei*; Nagase, Midori*; Arai, Tsuyoshi*; Emori, Tatsuya; Morita, Keisuke; Ban, Yasutoshi; Watanabe, So; Sano, Yuichi; Kunii, Shigeru*
no journal, ,
In this study, we investigated the adsorption characteristics of simulated nuclides of adsorbents carrying various NTA amide extracts. From the results of this test, It has become clear that HONTA-impregnated adsorbent showed a high adsorption distribution coefficient with respect to Ln (III) at a nitric acid concentration of 10 - 10
- 10 M. It has also revealed that all elements quickly reached adsorption equilibrium. In addition, the elution of the extractant and Sty / DVB was not occurred from the results of the extractant elution test from the impregnated adsorbent. We discuss the applicability of the extraction chromatography method using various NTA adsorbents.
M. It has also revealed that all elements quickly reached adsorption equilibrium. In addition, the elution of the extractant and Sty / DVB was not occurred from the results of the extractant elution test from the impregnated adsorbent. We discuss the applicability of the extraction chromatography method using various NTA adsorbents.