Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 separation and recovery technology; 3 Possibility of carbon dioxide separation using silica-based membranes and carbon dioxide reusing by membrane reactors
separation and recovery technology; 3 Possibility of carbon dioxide separation using silica-based membranes and carbon dioxide reusing by membrane reactorsNomura, Mikihiro*; Ishii, Katsunori; Sato, Yuya*
Recent Trends in Methanation and Green Hydrogen, p.148 - 157, 2023/10
no abstracts in English
Myagmarjav, O.; Shibata, Ai*; Tanaka, Nobuyuki; Noguchi, Hiroki; Kubo, Shinji; Nomura, Mikihiro*; Takegami, Hiroaki
International Journal of Hydrogen Energy, 46(56), p.28435 - 28449, 2021/08
Times Cited Count:2 Percentile:9.63(Chemistry, Physical)Myagmarjav, O.; Tanaka, Nobuyuki; Nomura, Mikihiro*; Noguchi, Hiroki; Imai, Yoshiyuki; Kamiji, Yu; Kubo, Shinji; Takegami, Hiroaki
Progress in Nuclear Energy, 137, p.103772_1 - 103772_7, 2021/07
Times Cited Count:6 Percentile:72.21(Nuclear Science & Technology)Tanaka, Nobuyuki; Sawada, Shinichi*; Yamaki, Tetsuya*; Kodaira, Takahide*; Kimura, Takehiro*; Nomura, Mikihiro*
Chemical Engineering Science, 237, p.116575_1 - 116575_11, 2021/06
Times Cited Count:1 Percentile:6.08(Engineering, Chemical)We have been developing the ion exchange membranes by a radiation grafted polymerization method to improve HI concentration performance for Electro-electrodialysis (EED) in the thermochemical water-splitting hydrogen production iodine-sulfur process. In this work, the crosslinking structures were introduced to the ion exchange membranes. The proton conductivity ( ), transport number (t
), transport number (t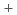 ), and water permeation factor (
), and water permeation factor ( ) of these crosslinked ion exchange membranes were measured and the effect of crosslinks to these performance indexes were investigated. The introduction of crosslinks was found to improve the selectivity of H
) of these crosslinked ion exchange membranes were measured and the effect of crosslinks to these performance indexes were investigated. The introduction of crosslinks was found to improve the selectivity of H and water transport (increase of t
and water transport (increase of t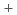 and decrease of
and decrease of  ), although the
), although the  somewhat decreased. The EED model that we established to discuss the permeation mechanism of EED system was used to theoretically analyze the effect of crosslink on the performance indexes. Based on this analysis of measurement results, the introduction of the crosslink was found to little affect the absorbed amount of HIx solution and H
somewhat decreased. The EED model that we established to discuss the permeation mechanism of EED system was used to theoretically analyze the effect of crosslink on the performance indexes. Based on this analysis of measurement results, the introduction of the crosslink was found to little affect the absorbed amount of HIx solution and H diffusion coefficient in the tested membranes, whereas it could lead to decrease I
diffusion coefficient in the tested membranes, whereas it could lead to decrease I diffusion coefficient. The results of
diffusion coefficient. The results of  and t
and t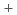 could reflect these effects. In addition, we found the fact that crosslink can inhibit the swelling due to the absorption of the HIx solution. As a result, the
could reflect these effects. In addition, we found the fact that crosslink can inhibit the swelling due to the absorption of the HIx solution. As a result, the  value decreased owing to the introduction of crosslink.
value decreased owing to the introduction of crosslink.
Sawada, Shinichi*; Kimura, Takehiro*; Nishijima, Haruyuki*; Kodaira, Takahide*; Tanaka, Nobuyuki; Kubo, Shinji; Imabayashi, Shinichiro*; Nomura, Mikihiro*; Yamaki, Tetsuya*
International Journal of Hydrogen Energy, 45(27), p.13814 - 13820, 2020/05
Times Cited Count:2 Percentile:6.8(Chemistry, Physical)An electrochemical membrane Bunsen reaction using a cation exchange membrane (CEM) is a key to achieving an iodine-sulfur (IS) thermochemical water splitting process for mass-production of hydrogen. In this study, we prepared both the radiation-grafted CEM with a high ion exchange capacity (IEC) and the highly-porous Au-electroplated anode, and then used them for the membrane Bunsen reaction to reduce the cell overvoltage. The high-IEC grafted CEM exhibited low resistivity for proton transport, while the porous Au anode had a large effective surface area for anodic SO oxidation reaction. As a result, the cell overvoltage for the membrane Bunsen reaction was significantly reduced to 0.21 V at 200 mA/cm
oxidation reaction. As a result, the cell overvoltage for the membrane Bunsen reaction was significantly reduced to 0.21 V at 200 mA/cm , which was only one-third of that of the previous test using the commercial CEM and non-porous anode. From the analysis of the current-voltage characteristics, employment of the grafted CEM was found to be more effective for the overvoltage reduction compared to the porous Au anode.
, which was only one-third of that of the previous test using the commercial CEM and non-porous anode. From the analysis of the current-voltage characteristics, employment of the grafted CEM was found to be more effective for the overvoltage reduction compared to the porous Au anode.
Myagmarjav, O.; Tanaka, Nobuyuki; Nomura, Mikihiro*; Kubo, Shinji
International Journal of Hydrogen Energy, 44(59), p.30832 - 30839, 2019/11
Times Cited Count:10 Percentile:33.23(Chemistry, Physical)Inagaki, Yoshiyuki; Sakaba, Nariaki; Tanaka, Nobuyuki; Nomura, Mikihiro*; Sawada, Shinichi*; Yamaki, Tetsuya*
Nihon Kaisui Gakkai-Shi, 73(4), p.194 - 202, 2019/08
The thermochemical IS process is a promising hydrogen production method which can produce hydrogen in a large amount and stably with high efficiency by thermal splitting of water. Research and development on chemical reaction technology with membranes was conducted for the purpose of improving the efficiency of IS process and application of solar heat. The basic technology of ceramic membranes applied to decomposition reactions of hydrogen iodine and sulfuric acid was developed, and it is expected that the conversion rate on decomposition in each reaction can be remarkably improved. The basic technology of a cation exchange membrane applied to Bunsen reaction was developed with radiation-induced grafting technique, it is expected that the amount of iodine can be reduced to about one-fifth compared to the conventional method. These achievements are important technologies for practical use of the IS process.
Myagmarjav, O.; Iwatsuki, Jin; Tanaka, Nobuyuki; Noguchi, Hiroki; Kamiji, Yu; Ioka, Ikuo; Kubo, Shinji; Nomura, Mikihiro*; Yamaki, Tetsuya*; Sawada, Shinichi*; et al.
International Journal of Hydrogen Energy, 44(35), p.19141 - 19152, 2019/07
Times Cited Count:16 Percentile:49.6(Chemistry, Physical)Myagmarjav, O.; Tanaka, Nobuyuki; Nomura, Mikihiro*; Kubo, Shinji
International Journal of Hydrogen Energy, 44(21), p.10207 - 10217, 2019/04
Times Cited Count:15 Percentile:47.35(Chemistry, Physical)Nomura, Mikihiro*; Kodaira, Takahide*; Ikeda, Ayumi*; Naka, Yasuhito*; Nishijima, Haruyuki*; Imabayashi, Shinichiro*; Sawada, Shinichi*; Yamaki, Tetsuya*; Tanaka, Nobuyuki; Kubo, Shinji
Journal of Chemical Engineering of Japan, 51(9), p.726 - 731, 2018/09
Times Cited Count:3 Percentile:13.13(Engineering, Chemical)Thermochemical hydrogen production by the iodine-sulfur process decomposes water into hydrogen and oxygen by combining the chemical reactions of iodine and sulfur. Two types of acids are produced through the Bunsen reaction. To improve the performance of this reaction, ion-exchange membranes for the membrane Bunsen reaction should be developed. In the present study, a cation-exchange membrane was prepared by using a radiation-graft polymerization method. It was found that a divinylbenzene crosslinking procedure was very effective in reducing water permeation through the membrane, and the membrane Bunsen reaction was successfully carried out by using the developed crosslinked membrane. Therefore, the developed crosslinked membrane is a potential candidate for cation-exchange membranes for the membrane Bunsen reaction.
Myagmarjav, O.; Tanaka, Nobuyuki; Nomura, Mikihiro*; Kubo, Shinji
International Journal of Hydrogen Energy, 42(49), p.29091 - 29100, 2017/12
Times Cited Count:20 Percentile:51.38(Chemistry, Physical)The catalytic decomposition of hydrogen iodide in a membrane reactor using silica membranes derived from hexyltrimethoxysilane (HTMOS) was investigated for the production of hydrogen in the thermochemical water splitting iodine-sulfur process. The silica membranes were prepared by counter-diffusion chemical vapor deposition using porous alumina support tubes in both the absence and presence of a  -alumina layer. The silica membranes formed on
-alumina layer. The silica membranes formed on  -alumina-coated
-alumina-coated  -alumina tubes displayed a higher H
-alumina tubes displayed a higher H permeance than that formed directly on an
permeance than that formed directly on an  -alumina tube. A silica membrane based on a 1.5
-alumina tube. A silica membrane based on a 1.5  m-thick
m-thick  -alumina layer fabricated under deposition conditions of 450
-alumina layer fabricated under deposition conditions of 450 C, 1200 s, and a N
C, 1200 s, and a N carrier gas velocity of 0.044 m s
carrier gas velocity of 0.044 m s exhibited a high H
exhibited a high H permeance of 9.4
permeance of 9.4  10
10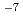 mol Pa
mol Pa m
m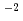 s
s while maintaining an H
while maintaining an H /N
/N selectivity of over 80.0. The performance of a membrane reactor based on an HTMOS-derived silica membrane was evaluated at 400
selectivity of over 80.0. The performance of a membrane reactor based on an HTMOS-derived silica membrane was evaluated at 400 C by measuring the HI conversion and H
C by measuring the HI conversion and H flow rates. The conversion was approximately 0.48 when the HI flow rate was 9.7 mL min
flow rates. The conversion was approximately 0.48 when the HI flow rate was 9.7 mL min .
.
 -permselective silica membrane for the separation of H
-permselective silica membrane for the separation of H from the hydrogen iodide decomposition reaction in the iodine-sulfur process
from the hydrogen iodide decomposition reaction in the iodine-sulfur processMyagmarjav, O.; Ikeda, Ayumi*; Tanaka, Nobuyuki; Kubo, Shinji; Nomura, Mikihiro*
International Journal of Hydrogen Energy, 42(9), p.6012 - 6023, 2017/03
Times Cited Count:19 Percentile:49.58(Chemistry, Physical)Kasahara, Seiji; Kubo, Shinji; Hino, Ryutaro; Onuki, Kaoru; Nomura, Mikihiro*; Nakao, Shinichi*
International Journal of Hydrogen Energy, 32(4), p.489 - 496, 2007/03
Times Cited Count:115 Percentile:91.83(Chemistry, Physical)Japan Atomic Energy Agency (JAEA) has been conducting the research and development on the thermochemical water-splitting IS process for effective hydrogen production using nuclear heat at temperatures close to 1000  C. Such temperatures can be supplied by High Temperature Gas-cooled Reactor (HTGR). JAEA's activity covers the studies on control of the process for continuous hydrogen production, the processing in HI decomposition procedure and a preliminary screening of corrosion resistant materials for the construction of the process. The present status of the study is hereby presented, with particular attention given to the studies of the flowsheet of the process using membranes for the HI processing.
C. Such temperatures can be supplied by High Temperature Gas-cooled Reactor (HTGR). JAEA's activity covers the studies on control of the process for continuous hydrogen production, the processing in HI decomposition procedure and a preliminary screening of corrosion resistant materials for the construction of the process. The present status of the study is hereby presented, with particular attention given to the studies of the flowsheet of the process using membranes for the HI processing.
Kasahara, Seiji; Onuki, Kaoru; Nomura, Mikihiro*; Nakao, Shinichi*
Journal of Chemical Engineering of Japan, 39(5), p.559 - 568, 2006/05
Times Cited Count:12 Percentile:42.39(Engineering, Chemical)Sensitivity analysis of the operation parameters and the chemical properties in the thermochemical hydrogen production IS process (iodine-sulfur process) was carried out for a static flow sheet. These parameters were evaluated by hydrogen production thermal efficiency, mass flow rate or heat exchange based on heat/mass balance. The most important parameters were concentration of HI after the electro-electrodialysis (EED) cell and apparent transport number of proton of the cation exchange membrane in the EED cell. HI concentration operation should be operated carefully because the parameters for optimum thermal efficiency and for optimum flow rate and heat exchange were different. For the chemical properties, composition at the inlet of the HI decomposition procedure and HIx pseudo-azeotropic composition had great effects. HI concentration after the EED cell should be optimized for each composition. Order of priority for assessment of the operation parameters and chemical properties was determined by the evaluation.
Nomura, Mikihiro*; Okuda, Hiroyuki; Kasahara, Seiji; Nakao, Shinichi*
Chemical Engineering Science, 60(24), p.7160 - 7167, 2005/12
Times Cited Count:29 Percentile:66.02(Engineering, Chemical)The Bunsen reaction (SO +I
+I +2H
+2H O=H
O=H SO
SO +2HI) in the water-splitting IS process was examined using an electrochemical cell with a cation exchange membrane. The optimal molalities of anolyte and catholyte were evaluated by total thermal efficiency. The I
+2HI) in the water-splitting IS process was examined using an electrochemical cell with a cation exchange membrane. The optimal molalities of anolyte and catholyte were evaluated by total thermal efficiency. The I /HI ratio had little effect on the required total voltage; the I
/HI ratio had little effect on the required total voltage; the I /HI ratio can be reduced to 0.5 without decreasing the efficiency. HI and H
/HI ratio can be reduced to 0.5 without decreasing the efficiency. HI and H SO
SO molality greatly affected the efficiency. Membrane resistances are very important parameters affecting the efficiency. The total thermal efficiency increased by 3.0% by increasing the operating temperature from 313K to 363K.
molality greatly affected the efficiency. Membrane resistances are very important parameters affecting the efficiency. The total thermal efficiency increased by 3.0% by increasing the operating temperature from 313K to 363K.
Kasahara, Seiji; Kubo, Shinji; Hino, Ryutaro; Onuki, Kaoru; Nomura, Mikihiro*; Nakao, Shinichi*
Proceedings of AIChE 2005 Spring National Meeting (CD-ROM), 8 Pages, 2005/04
Japan Atomic Energy Research Institute (JAERI) has been conducting the research and development on the thermochemical water-splitting IS process for effective hydrogen production using nuclear heat of close to 1000  C that can be supplied from High Temperature Gas-cooled Reactor (HTGR). The activity covers the studies on the process control for the continuous hydrogen production, the process improvements in the HI decomposition procedure and the preliminary screening of corrosion resistant materials of construction. Present status of the study is presented, especially, focusing on the process flowsheeting study concerning the application of membrane process for the HI processing.
C that can be supplied from High Temperature Gas-cooled Reactor (HTGR). The activity covers the studies on the process control for the continuous hydrogen production, the process improvements in the HI decomposition procedure and the preliminary screening of corrosion resistant materials of construction. Present status of the study is presented, especially, focusing on the process flowsheeting study concerning the application of membrane process for the HI processing.
Kasahara, Seiji; Hwang, G.*; Nakajima, Hayato; Choi, H.*; Onuki, Kaoru; Nomura, Mikihiro
Journal of Chemical Engineering of Japan, 36(7), p.887 - 899, 2003/07
Times Cited Count:66 Percentile:87.93(Engineering, Chemical)Thermal efficiency of the IS thermochemical hydrogen production process was evaluated. Sensitivities of operation conditions (HI conversion ratio, pressure and reflux ratio at HI distillation and concentration of HI after EED) and nonidealities of the process (electric energy loss in EED, loss at heat exchangers and loss of waste heat recovery as electricity) were investigated. Concentration of HI after EED had the most significant effect of 13.3 % on thermal efficiency in operation conditions. Nonidealities had importance on thermal efficiency. Thermal efficiency was 56.8 % with optimized operation conditions and no nonidealities.
Nomura, Mikihiro; Kasahara, Seiji; Onuki, Kaoru
JAERI-Research 2002-039, 24 Pages, 2003/01
Thermal efficiency to produce hydrogen from water through the IS process was evaluated by a viewpoint of thermodynamics. Thermal efficiency is decided by a temperature from a heat source and limited by the works calculated by the Carnot efficiency for any hydrogen production methods. The maximum thermal efficiency is 81.3% for a thermal cycle between 1123K and 733K. The thermal efficiency of the IS process was evaluated by G-T diagrams of each reactions and separation processes. The maximum value is 78.2% without considering the works for separations of acids from water. However, the effects of the works for separations on thermal efficiency are essential for the IS process, because Gibbs energies of separations of acids from water are always positive. The thermal efficiency could be changed from 53.5% to 76.6% by the calculation with or without the separation processes.
Kodaira, Takahide; Ikeda, Ayumi*; Matsuyama, Emi*; Kono, Nobuho*; Sawada, Shinichi; Yamaki, Tetsuya; Nomura, Mikihiro*
no journal, ,
In the thermochemical water splitting IS process, the Bunsen reaction (SO + I
+ I + 2H
+ 2H O = H
O = H SO
SO + 2HI) needs to be achieved in an electrochemical cell with an ion exchange membrane, which renders separation procedures unnecessary. As part of a JST-ALCA project, therefore, we have been developing ion exchange membranes for this application by using methods of radiation crosslinking and/or radiation graft polymerization. Our preliminary experiments made it possible to confirm controllability of the degree of grafting,
+ 2HI) needs to be achieved in an electrochemical cell with an ion exchange membrane, which renders separation procedures unnecessary. As part of a JST-ALCA project, therefore, we have been developing ion exchange membranes for this application by using methods of radiation crosslinking and/or radiation graft polymerization. Our preliminary experiments made it possible to confirm controllability of the degree of grafting, 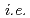 , ion exchange capacity by varying the conditions of the grafting.
, ion exchange capacity by varying the conditions of the grafting.
Kodaira, Takahide; Ikeda, Ayumi*; Matsuyama, Emi*; Kono, Nobuho*; Sawada, Shinichi; Yamaki, Tetsuya; Nomura, Mikihiro*
no journal, ,
In the thermochemical water splitting IS process, the Bunsen reaction (SO + I
+ I + 2H
+ 2H O = H
O = H SO
SO + 2HI) needs to be achieved in an electrochemical cell with an ion exchange membrane, which renders separation procedures unnecessary. As part of a JST-ALCA project, therefore, we have been developing ion exchange membranes for this application by using methods of radiation crosslinking and/or radiation graft polymerization. Our preliminary experiments made it possible to confirm controllability of the degree of grafting,
+ 2HI) needs to be achieved in an electrochemical cell with an ion exchange membrane, which renders separation procedures unnecessary. As part of a JST-ALCA project, therefore, we have been developing ion exchange membranes for this application by using methods of radiation crosslinking and/or radiation graft polymerization. Our preliminary experiments made it possible to confirm controllability of the degree of grafting, 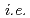 , ion exchange capacity by varying the conditions of the grafting. Importantly, controlling the fixed-charge density of the membrane should both lower permeability of SO
, ion exchange capacity by varying the conditions of the grafting. Importantly, controlling the fixed-charge density of the membrane should both lower permeability of SO and enhance the transport number of H
and enhance the transport number of H .
.