Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Cl dissociation, CH
Cl dissociation, CH abstraction, and Cl adsorption from the dissociative scattering of supersonic CH
abstraction, and Cl adsorption from the dissociative scattering of supersonic CH Cl on Cu(111) and Cu(410)
Cl on Cu(111) and Cu(410)Makino, Takamasa*; Tsuda, Yasutaka; Yoshigoe, Akitaka; Di o, W. A.*; Okada, Michio*
o, W. A.*; Okada, Michio*
Applied Surface Science, 642, p.158568_1 - 158568_6, 2024/01
Times Cited Count:0 Percentile:0.01(Chemistry, Physical)Tsuda, Yasutaka; Gueriba, J. S.*; Ueta, Hirokazu*; Di o, W. A.*; Kurahashi, Mitsunori*; Okada, Michio*
o, W. A.*; Kurahashi, Mitsunori*; Okada, Michio*
JACS Au (Internet), 2(8), p.1839 - 1847, 2022/08
 molecular adsorption on Cu(111); Copper oxides
molecular adsorption on Cu(111); Copper oxidesHayashida, Koki*; Tsuda, Yasutaka; Yamada, Takashi*; Yoshigoe, Akitaka; Okada, Michio*
ACS Omega (Internet), 6(40), p.26814 - 26820, 2021/10
Times Cited Count:7 Percentile:44.69(Chemistry, Multidisciplinary)We report the X-ray photoemission spectroscopy (XPS) characterization of the bulk Cu O(111) surface and 8-type and 29-type oxide structures on Cu(111) prepared by using 0.5 eV O
O(111) surface and 8-type and 29-type oxide structures on Cu(111) prepared by using 0.5 eV O supersonic molecular beam (SSMB) source. We propose a new structural model for the 8-type oxide structure and also confirmed the previously proposed model for the [29] oxide structure on Cu(111), based on the O1s XPS spectra. The detection-angle dependence of the O 1s spectra supports that the nanopyramidal model is more preferable for the (
supersonic molecular beam (SSMB) source. We propose a new structural model for the 8-type oxide structure and also confirmed the previously proposed model for the [29] oxide structure on Cu(111), based on the O1s XPS spectra. The detection-angle dependence of the O 1s spectra supports that the nanopyramidal model is more preferable for the ( X
X )R30
)R30 Cu
Cu O(111). We also report the electronic excitations which O1s electrons suffer.
O(111). We also report the electronic excitations which O1s electrons suffer.
 O formation on Cu
O formation on Cu Pd(111) and Cu
Pd(111) and Cu Pt(111)
Pt(111)Tsuda, Yasutaka; Gueriba, J. S.*; Makino, Takamasa*; Di o, W. A.*; Yoshigoe, Akitaka; Okada, Michio*
o, W. A.*; Yoshigoe, Akitaka; Okada, Michio*
Scientific Reports (Internet), 11, p.3906_1 - 3906_8, 2021/02
Times Cited Count:3 Percentile:16.03(Multidisciplinary Sciences)Okada, Michio*; Tsuda, Yasutaka*; Yoshigoe, Akitaka; Di o, W. A.*
o, W. A.*
Do To Dogokin, 56(1), p.232 - 236, 2017/00
We reported the our studies on the surface temperature (Ts) dependence of oxidation on the Cu Au(111) surface by a supersonic O
Au(111) surface by a supersonic O molecular beam, using synchrotron radiation X-ray photoemission spectroscopy. Clean surface shows strong Au segregation to the top layer, i.e., Au surface enrichment of the clean surface. Complete Cu segregation to the surface occurs at 0.5 ML O surface coverage. The Au-rich second and third layers of the oxidized surface demonstrate the protective layer formation against oxidation deeper into the bulk. We found that Cu
molecular beam, using synchrotron radiation X-ray photoemission spectroscopy. Clean surface shows strong Au segregation to the top layer, i.e., Au surface enrichment of the clean surface. Complete Cu segregation to the surface occurs at 0.5 ML O surface coverage. The Au-rich second and third layers of the oxidized surface demonstrate the protective layer formation against oxidation deeper into the bulk. We found that Cu O formation occurs. At Ts = 300K, the Cu
O formation occurs. At Ts = 300K, the Cu O growth is not so effective. The surface oxidation is less effective on Cu
O growth is not so effective. The surface oxidation is less effective on Cu Au(111) than on Cu(111). At Ts = 400K, the protection by the Au-rich layer against oxidation into bulk is effective. At Ts = 500K, the Au protective layer is broken due to effective Au diffusion and thus Cu
Au(111) than on Cu(111). At Ts = 400K, the protection by the Au-rich layer against oxidation into bulk is effective. At Ts = 500K, the Au protective layer is broken due to effective Au diffusion and thus Cu O grows deeper into bulk.
O grows deeper into bulk.
Okada, Michio*; Tsuda, Yasutaka*; Oka, Kohei*; Kojima, Kazuki*; Di o, W. A.*; Yoshigoe, Akitaka; Kasai, Hideaki*
o, W. A.*; Yoshigoe, Akitaka; Kasai, Hideaki*
Scientific Reports (Internet), 6, p.31101_1 - 31101_8, 2016/08
Times Cited Count:28 Percentile:73.49(Multidisciplinary Sciences)We report results of our experimental and theoretical studies on the oxidation of Cu-Au alloy surfaces, viz., Cu Au(111), CuAu(111), and Au
Au(111), CuAu(111), and Au Cu(111), using hyperthermal O
Cu(111), using hyperthermal O molecular beam (HOMB). We observed strong Au segregation to the top layer of the corresponding clean (111) surfaces. This forms a protective layer that hinders further oxidation into the bulk. The higher the concentration of Au in the protective layer formed, the higher the protective efficacy. As a result, of the three Cu-Au surfaces studied, Au
molecular beam (HOMB). We observed strong Au segregation to the top layer of the corresponding clean (111) surfaces. This forms a protective layer that hinders further oxidation into the bulk. The higher the concentration of Au in the protective layer formed, the higher the protective efficacy. As a result, of the three Cu-Au surfaces studied, Au Cu(111) is the most stable against dissociative adsorption of O
Cu(111) is the most stable against dissociative adsorption of O , even with HOMB. We also found that this protective property breaks down for oxidations occurring at temperatures above 300 K.
, even with HOMB. We also found that this protective property breaks down for oxidations occurring at temperatures above 300 K.
 Au(111) by an energetic oxygen molecule
Au(111) by an energetic oxygen moleculeTsuda, Yasutaka*; Yoshigoe, Akitaka; Teraoka, Yuden; Okada, Michio*
Materials Research Express (Internet), 3(3), p.035014_1 - 035014_8, 2016/03
Times Cited Count:5 Percentile:18.8(Materials Science, Multidisciplinary)We report a study on the surface-temperature (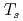 ) dependence of oxidation process at Cu
) dependence of oxidation process at Cu Au(111) by using a hyperthermal oxygen molecular beam and synchrotron-radiation X-ray photoemission spectroscopy. The O-1s spectra and the corresponding O-uptake curves demonstrate that Cu
Au(111) by using a hyperthermal oxygen molecular beam and synchrotron-radiation X-ray photoemission spectroscopy. The O-1s spectra and the corresponding O-uptake curves demonstrate that Cu O domains grow effectively at high
O domains grow effectively at high 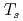 of 400 and 500 K. The simple analysis of the O distribution suggests that the temperature-induced atomic diffusion causes the Cu
of 400 and 500 K. The simple analysis of the O distribution suggests that the temperature-induced atomic diffusion causes the Cu O domains growing thicker at 500 K. The oxidation of Cu
O domains growing thicker at 500 K. The oxidation of Cu Au(111) is less efficient at
Au(111) is less efficient at 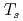 = 300-500 K than that of Cu(111), demonstrating that the protective nature of Cu
= 300-500 K than that of Cu(111), demonstrating that the protective nature of Cu Au against oxidation, in comparison to Cu, remains even at high
Au against oxidation, in comparison to Cu, remains even at high 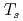 .
.
 Au(111)
Au(111)Oka, Kohei*; Tsuda, Yasutaka*; Makino, Takamasa*; Okada, Michio*; Hashinokuchi, Michihiro*; Yoshigoe, Akitaka; Teraoka, Yuden; Kasai, Hideaki*
Physical Chemistry Chemical Physics, 16(36), p.19702 - 19711, 2014/08
Times Cited Count:11 Percentile:40.33(Chemistry, Physical) Au(111) oxidation; Oxygen induced Cu segregation and the protective Au layer profile
Au(111) oxidation; Oxygen induced Cu segregation and the protective Au layer profileTsuda, Yasutaka*; Oka, Kohei*; Makino, Takamasa*; Okada, Michio*; Di o, W. A.*; Hashinokuchi, Michihiro*; Yoshigoe, Akitaka; Teraoka, Yuden; Kasai, Hideaki*
o, W. A.*; Hashinokuchi, Michihiro*; Yoshigoe, Akitaka; Teraoka, Yuden; Kasai, Hideaki*
Physical Chemistry Chemical Physics, 16(8), p.3815 - 3822, 2014/02
Times Cited Count:14 Percentile:49(Chemistry, Physical) O formation on Cu
O formation on Cu Au(110) surface with energetic O
Au(110) surface with energetic O molecular beams
molecular beamsHashinokuchi, Michihiro*; Yoshigoe, Akitaka; Teraoka, Yuden; Okada, Michio*
Applied Surface Science, 287, p.282 - 286, 2013/12
Times Cited Count:6 Percentile:29.39(Chemistry, Physical)Hashinokuchi, Michihiro*; Tode, Mayumi*; Yoshigoe, Akitaka; Teraoka, Yuden; Okada, Michio*
Applied Surface Science, 276, p.276 - 283, 2013/07
Times Cited Count:4 Percentile:20.56(Chemistry, Physical) O formation on a Cu(110) surface with an energetic O
O formation on a Cu(110) surface with an energetic O molecular beam
molecular beamHashinokuchi, Michihiro*; Yoshigoe, Akitaka; Teraoka, Yuden; Okada, Michio*
Journal of Physics; Condensed Matter, 24(39), p.395007_1 - 395007_8, 2012/10
Times Cited Count:5 Percentile:23.98(Physics, Condensed Matter) molecular beam; Cu(511) vs Cu(410)
molecular beam; Cu(511) vs Cu(410)Okada, Michio*; Vattuone, L.*; Rocca, M.*; Teraoka, Yuden
Journal of Chemical Physics, 136(9), p.094704_1 - 094704_8, 2012/03
Times Cited Count:8 Percentile:28.11(Chemistry, Physical) Au with synchrotron radiation
Au with synchrotron radiationSowa, Makoto*; Yamazaki, Daichi*; Okada, Michio*; Yoshigoe, Akitaka; Teraoka, Yuden; Kasai, Toshio*
Electrical Engineering in Japan, 175(4), p.43 - 47, 2011/06
Times Cited Count:2 Percentile:18.52(Engineering, Electrical & Electronic)Yamazaki, Daichi*; Sowa, Makoto*; Okada, Michio*; Teraoka, Yuden; Kasai, Toshio*
Journal of the Vacuum Society of Japan, 54(5), p.307 - 312, 2011/05
Okada, Michio*; Sowa, Makoto*; Kasai, Toshio*; Teraoka, Yuden
Applied Surface Science, 257(9), p.4257 - 4263, 2011/02
Times Cited Count:11 Percentile:44.66(Chemistry, Physical)Hashinokuchi, Michihiro*; Sumimoto, Yuichi*; Tode, Mayumi; Harries, J.; Okada, Michio*; Teraoka, Yuden; Kasai, Toshio*
Denki Gakkai Rombunshi, C, 130(10), p.1723 - 1729, 2010/10
The oxidation processes on a TiAl surface induced by a hyperthermal O molecular beam (HOMB) with a translational energy of 2.2 eV was studied by X-ray photoemission spectroscopy in conjunction with synchrotron radiation. At a surface temperature of 300 K, the simultaneous growth of Al and Ti oxides accompanied with the segregation of Al
molecular beam (HOMB) with a translational energy of 2.2 eV was studied by X-ray photoemission spectroscopy in conjunction with synchrotron radiation. At a surface temperature of 300 K, the simultaneous growth of Al and Ti oxides accompanied with the segregation of Al O
O near the surface was observed. The efficiency of oxidation for the HOMB incidence was smaller than that for O
near the surface was observed. The efficiency of oxidation for the HOMB incidence was smaller than that for O backfilling (25 meV). Furthermore, the chemical compositions of oxide species (Al
backfilling (25 meV). Furthermore, the chemical compositions of oxide species (Al O
O , Ti
, Ti O
O , TiO
, TiO ) on the TiAl surface were independent of the translational energy of incident O
) on the TiAl surface were independent of the translational energy of incident O molecule. The present results suggest that the oxidation on TiAl surface proceeds via precursor molecular states.
molecule. The present results suggest that the oxidation on TiAl surface proceeds via precursor molecular states.
 Au(110) using hyperthermal O
Au(110) using hyperthermal O molecular beam
molecular beamOkada, Michio*; Teraoka, Yuden
Applied Surface Science, 256(18), p.5676 - 5680, 2010/07
Times Cited Count:8 Percentile:37.46(Chemistry, Physical)Oxidation of Cu Au(110) using a hyperthermal O
Au(110) using a hyperthermal O molecular beam (HOMB) was investigated by synchrotron X-ray photoemission spectroscopy. From the incident energy dependence of the O-uptake curve, the precursor-mediated dissociative adsorption occurs, where the trapped O
molecular beam (HOMB) was investigated by synchrotron X-ray photoemission spectroscopy. From the incident energy dependence of the O-uptake curve, the precursor-mediated dissociative adsorption occurs, where the trapped O molecule can migrate and dissociate at the lower activation-barrier sites. Dissociative adsorption of O
molecule can migrate and dissociate at the lower activation-barrier sites. Dissociative adsorption of O on Cu
on Cu Au(110) is as effective at the thermal O
Au(110) is as effective at the thermal O exposure as on Cu(110). On the other hand, at the incident energies of HOMB where the direct dissociative adsorption is dominant, the dissociative adsorption of O
exposure as on Cu(110). On the other hand, at the incident energies of HOMB where the direct dissociative adsorption is dominant, the dissociative adsorption of O takes place with a higher activation barrier and less reactivity due to Au alloying comparing to the HOMB oxidation of Cu(110). The dissociative adsorption progresses with the Cu segregation on Cu
takes place with a higher activation barrier and less reactivity due to Au alloying comparing to the HOMB oxidation of Cu(110). The dissociative adsorption progresses with the Cu segregation on Cu Au(110) similarly as on Cu
Au(110) similarly as on Cu Au(100). The growth of Cu
Au(100). The growth of Cu O for 2 eV HOMB suggests that the diffusion of Cu also contribute to the oxidation.
O for 2 eV HOMB suggests that the diffusion of Cu also contribute to the oxidation.
 molecular beams
molecular beamsOkada, Michio*; Moritani, Kosuke*; Vattuone, L.*; Savio, L.*; Teraoka, Yuden; Kasai, Toshio*; Rocca, M.*
Metal Oxide Nanostructures and Their Applications, 1, p.205 - 237, 2010/03
The use of hyperthermal O molecular beams may improve the quality of thin film growth, for example, for organic films, and allow the production of oxide layers at lower crystal temperatures, avoiding contamination problems and reducing film defects. Collision-induced absorption and local heating of the substrate were shown to be indeed effective in inducing oxide nucleation, opening up new possibilities for the production of nanostructured metal oxides. Herein we offer an overview on recent detailed studies of oxygen adsorption and of the initial stages of Cu
molecular beams may improve the quality of thin film growth, for example, for organic films, and allow the production of oxide layers at lower crystal temperatures, avoiding contamination problems and reducing film defects. Collision-induced absorption and local heating of the substrate were shown to be indeed effective in inducing oxide nucleation, opening up new possibilities for the production of nanostructured metal oxides. Herein we offer an overview on recent detailed studies of oxygen adsorption and of the initial stages of Cu O and CuO formation on low Miller index and vicinal Cu surfaces. We introduce the hyperthermal molecular beam technique and give some details on the experimental apparatuses. We discuss the available data for Cu(100), Cu(410) and Cu(110) and Cu(111), respectively.
O and CuO formation on low Miller index and vicinal Cu surfaces. We introduce the hyperthermal molecular beam technique and give some details on the experimental apparatuses. We discuss the available data for Cu(100), Cu(410) and Cu(110) and Cu(111), respectively.
Moritani, Kosuke*; Okada, Michio*; Teraoka, Yuden; Yoshigoe, Akitaka; Kasai, Toshio*
Journal of Physical Chemistry A, 113(52), p.15217 - 15222, 2009/10
Times Cited Count:11 Percentile:35.59(Chemistry, Physical)Oxygen adsorption and subsequent oxide formation on Cu(110) using a hyperthermal oxygen molecular beam (HOMB) has been investigated using SR-XPS. The O-uptake curves, which were determined from the evolution of O-1s peaks, indicate that simple Langmuir type kinetics can describe dissociative adsorption of O with an incident energy (Ei) below 0.5eV under oxygen coverage of 0.5 ML. The reaction order dependence on Ei implies two competing dissociation mechanisms, trapping-mediated and directly-activated adsorption. Oxidation at oxygen coverage larger than 0.5 ML proceeds rather effectively using highly energetic HOMB at Ei larger than 1.0 eV. The surface Cu
with an incident energy (Ei) below 0.5eV under oxygen coverage of 0.5 ML. The reaction order dependence on Ei implies two competing dissociation mechanisms, trapping-mediated and directly-activated adsorption. Oxidation at oxygen coverage larger than 0.5 ML proceeds rather effectively using highly energetic HOMB at Ei larger than 1.0 eV. The surface Cu O formed with highly-energetic HOMB incidence decomposes with desorbing subsurface oxygen even at room temperature. This indicates that HOMB can induce a meta stable surface structure that cannot be produced in the thermal equilibrium process.
O formed with highly-energetic HOMB incidence decomposes with desorbing subsurface oxygen even at room temperature. This indicates that HOMB can induce a meta stable surface structure that cannot be produced in the thermal equilibrium process.