Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Okamura, Hiroyuki
Nihon Ion Kokan Gakkai-Shi, 35(1), p.1 - 8, 2024/01
Ionic liquids have unique properties that are quite different from conventional molecular liquids, and have therefore attracted much attention in various fields as novel functional media. When ionic liquids are used as extraction media in solvent extraction, they can also act as "liquid ion exchangers", and it is thus expected to exhibit superior extractability and separability compared with those in conventional organic solvent systems. The author has focused on these characteristics of ionic liquids, and have studied on the extraction separation of metal ions using ionic liquids as extraction media, with the aim of developing highly efficient solvent extraction systems. This article presents the evaluation of the solvent properties of ionic liquids in metal ion extraction, the separation of rare earth metals by the synergistic effect in ionic liquid systems, and the development of a macrocyclic extractant and the structure analysis of the metal complex extracted into the ionic liquid.
Kinoshita, Takahiro*; Okamura, Shigeki*; Nishino, Hiroyuki; Yamano, Hidemasa; Kurisaka, Kenichi; Futagami, Satoshi; Fukasawa, Tsuyoshi*
Transactions of 26th International Conference on Structural Mechanics in Reactor Technology (SMiRT-26) (Internet), 7 Pages, 2022/07
The seismic evaluation of key components such as reactor vessel is important for the Seismic Probabilistic Risk Assessment (S-PRA) in a Sodium-Cooled Fast Reactor (SFR). Many components were damaged by cumulative damage like fatigue damage during seismic ground motion. However, general evaluation method for key components under seismic ground motion has been based on static loads and elastic region of materials. More accurate evaluation method for S-PRA, which can evaluate the failure of key components such as reactor vessels, has been actually required. In this study, failure probability evaluation method with integrated energy was developed by comparing the energy with vibration tests and fatigue tests. Vibration tests were performed to evaluate integrated vibration energy at failure by energy balance equation and fatigue tests were performed to evaluate integrated vibration energy at failure based on experimental results of fatigue tests.
Ueda, Yuki; Eguchi, Ayano; Tokunaga, Kohei; Kikuchi, Kei*; Sugita, Tsuyoshi; Okamura, Hiroyuki; Naganawa, Hirochika
Industrial & Engineering Chemistry Research, 61(19), p.6640 - 6649, 2022/05
Times Cited Count:1 Percentile:12.67(Engineering, Chemical)no abstracts in English
Okamura, Hiroyuki; Hirayama, Naoki*
Analytical Sciences, 37(1), p.119 - 130, 2021/01
Times Cited Count:30 Percentile:77(Chemistry, Analytical)This review summarizes recent progress in solvent extraction of rare earth elements (REEs) using an ionic liquid (IL) as the extraction solvent. These IL extraction systems are advantageous owing to the affinity of ILs for both charged and neutral hydrophobic species, in contrast to conventional organic solvent extraction systems. Herein, REE extraction studies using ILs are detailed and classified based on the type of extraction system. In IL extraction systems, the extracted complexes are often different from those in organic solvent systems, and the REE extraction and separation efficiencies are often significantly enhanced. Synergistic IL extraction is an effective approach to improving the extractability and separability of REEs. The development of novel task-specific ionic liquids (TSILs) suitable for IL extraction systems is also effective for REE separation.
 from nitrate media; Comparison with a conventional organic phosphate
from nitrate media; Comparison with a conventional organic phosphateUeda, Yuki; Kikuchi, Kei*; Tokunaga, Kohei; Sugita, Tsuyoshi; Aoyagi, Noboru; Tanaka, Kazuya; Okamura, Hiroyuki
Solvent Extraction and Ion Exchange, 39(5-6), p.491 - 511, 2021/00
Times Cited Count:0 Percentile:0(Chemistry, Multidisciplinary)no abstracts in English
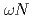 scattering length from
scattering length from  photoproduction on the proton near the reaction threshold
photoproduction on the proton near the reaction thresholdIshikawa, Takatsugu*; Fujimura, Hisako*; Fukasawa, Hiroshi*; Hashimoto, Ryo*; He, Q.*; Honda, Yuki*; Hosaka, Atsushi; Iwata, Takahiro*; Kaida, Shun*; Kasagi, Jirota*; et al.
Physical Review C, 101(5), p.052201_1 - 052201_6, 2020/05
Times Cited Count:4 Percentile:45.12(Physics, Nuclear)Okamura, Hiroyuki; Mizuno, Masayoshi*; Hirayama, Naoki*; Shimojo, Kojiro; Naganawa, Hirochika; Imura, Hisanori*
Industrial & Engineering Chemistry Research, 59(1), p.329 - 340, 2020/01
Times Cited Count:15 Percentile:57.71(Engineering, Chemical)The synergistic ionic-liquid extraction and extraction equilibrium of lanthanoid(III) (Ln(III)) ions have been investigated using 2-thenoyltrifluoroacetone (Htta) and trioctylphosphine oxide (TOPO) in the ionic liquid 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C mim][Tf
mim][Tf N]). The selective synergistic effect for heavier Ln(III) ions was found using a combination of Htta and TOPO in [C
N]). The selective synergistic effect for heavier Ln(III) ions was found using a combination of Htta and TOPO in [C mim][Tf
mim][Tf N], leading to enhanced separability among Ln(III) ions. The extracted Ln(III) species and the extraction constants in the Htta-TOPO system were determined by three-dimensional extraction equilibrium analysis. The selective synergism for heavier Ln(III) ions in the Htta-TOPO system was ascribed to the formation of hydrophobic, charged adducts, such as Ln(tta)
N], leading to enhanced separability among Ln(III) ions. The extracted Ln(III) species and the extraction constants in the Htta-TOPO system were determined by three-dimensional extraction equilibrium analysis. The selective synergism for heavier Ln(III) ions in the Htta-TOPO system was ascribed to the formation of hydrophobic, charged adducts, such as Ln(tta) (TOPO)
(TOPO)
 and Ln(tta)(TOPO)
and Ln(tta)(TOPO)
 , in [C
, in [C mim][Tf
mim][Tf N].
N].
 O(
O( He,
He, )
) F reaction; Identification of the low-energy "super" -Gamow-Teller state
F reaction; Identification of the low-energy "super" -Gamow-Teller stateFujita, Hirohiko*; Fujita, Yoshitaka*; Utsuno, Yutaka; Yoshida, Kenichi*; Adachi, Tatsuya*; Algora, A.*; Csatl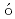 s, M.*; Deaven, J. M.*; Estevez-Aguado, E.*; Guess, C. J.*; et al.
s, M.*; Deaven, J. M.*; Estevez-Aguado, E.*; Guess, C. J.*; et al.
Physical Review C, 100(3), p.034618_1 - 034618_13, 2019/09
Times Cited Count:12 Percentile:77.09(Physics, Nuclear)no abstracts in English
Atanassova, M.*; Okamura, Hiroyuki; Eguchi, Ayano; Ueda, Yuki; Sugita, Tsuyoshi; Shimojo, Kojiro
Analytical Sciences, 34(8), p.973 - 978, 2018/08
Times Cited Count:17 Percentile:59.57(Chemistry, Analytical)The distribution constants of 4-benzoyl-3-phenyl-5-isoxazolone (HPBI) and deprotonated one (PBI ) between hydrophobic ionic liquid 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C
) between hydrophobic ionic liquid 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C C
C im][Tf
im][Tf N]) and aqueous phases were determined, together with the acid-dissociation constant of HPBI. The solvent extraction of three selected lanthanoid ions (La
N]) and aqueous phases were determined, together with the acid-dissociation constant of HPBI. The solvent extraction of three selected lanthanoid ions (La , Eu
, Eu , and Lu
, and Lu ) with HPBI from aqueous nitrate phase into [C
) with HPBI from aqueous nitrate phase into [C C
C im][Tf
im][Tf N] has been investigated. Application of the ionic liquid as the extracting phase greatly enhanced the extraction performance of HPBI for lanthanoid ions compared with that in the chloroform system. The composition of the extracted species was established to be anionic tetrakis entities, Ln(PBI)
N] has been investigated. Application of the ionic liquid as the extracting phase greatly enhanced the extraction performance of HPBI for lanthanoid ions compared with that in the chloroform system. The composition of the extracted species was established to be anionic tetrakis entities, Ln(PBI)
 , for light, middle, and heavy lanthanoid ions in an ionic environment.
, for light, middle, and heavy lanthanoid ions in an ionic environment.
Okamura, Hiroyuki
Kagaku To Kyoiku, 66(6), p.286 - 287, 2018/06
The emulsion flow method has recently been developed and is an extraction technique based on the liquid-liquid system. This method has attracted much attention as an innovative technique combining simplicity and high performance. The emulsion flow apparatus can actualize solvent extraction by only solution sending. In this article, mechanism of the emulsion flow method was explained and recovery and removal of uranium was introduced as an application example.
Hatakeyama, Mizuki*; Nishiyama, Yoshio*; Nagatani, Hirohisa*; Okamura, Hiroyuki; Imura, Hisanori*
Solvent Extraction Research and Development, Japan, 25(2), p.79 - 89, 2018/00
Times Cited Count:12 Percentile:42.69(Chemistry, Multidisciplinary)The synergistic extraction of trivalent lanthanide ions (Ln(III)) with benzoylacetone (Hba) and trioctyphosphine oxide (TOPO) in an ionic liquid (IL), 1-butyl-3-methyl-imidazolium bis(trifluoromethanesulfonyl)imide ([Bmim][Tf N]), has been investigated. The extractability of Ln(III) with Hba was significantly enhanced in the presence of TOPO. The composition and extraction constants of the extracted species for each Ln(III) were determined by 3-dimensional equilibrium analysis. It was found that all Ln(III) were extracted as cationic ternary complexes such as Ln(ba)
N]), has been investigated. The extractability of Ln(III) with Hba was significantly enhanced in the presence of TOPO. The composition and extraction constants of the extracted species for each Ln(III) were determined by 3-dimensional equilibrium analysis. It was found that all Ln(III) were extracted as cationic ternary complexes such as Ln(ba) (TOPO)
(TOPO)
 or Ln(ba)(TOPO)
or Ln(ba)(TOPO)
 with Hba and TOPO. Furthermore, the formation constants of the cationic ternary complexes in the IL indicate that Lu(ba)(TOPO)
with Hba and TOPO. Furthermore, the formation constants of the cationic ternary complexes in the IL indicate that Lu(ba)(TOPO)
 is the most stable complex in the IL.
is the most stable complex in the IL.
Okamura, Hiroyuki
Bunseki Kagaku, 66(7), p.531 - 532, 2017/07
This paper summarizes the author's doctoral thesis on analytical chemistry. In this study, the ionic liquid (IL) extraction of various metal(II, III) complexes with macrocyclic and anionic chelating ligands has been investigated to clarify the solvent effect of ILs and to demonstrate the specificity and superiority of the IL extraction systems over conventional ones. The evaluation of the extraction equilibrium of Eu(III) with 2-thenoyltrifluoroacetone (Htta) in 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C mim][Tf
mim][Tf N]) and time-resolved laser-induced fluorescence spectroscopy revealed the specific solute-solvent interactions between Eu(tta)
N]) and time-resolved laser-induced fluorescence spectroscopy revealed the specific solute-solvent interactions between Eu(tta) and [C
and [C mim][Tf
mim][Tf N]. The IL synergistic extraction with Htta and
N]. The IL synergistic extraction with Htta and 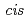 -dicyclohexano-18-crown-6 was developed. The extractability of lighter lanthanides(III) was remarkably enhanced by a synergistic effect of crown ethers. A macrocyclic ligand (H
-dicyclohexano-18-crown-6 was developed. The extractability of lighter lanthanides(III) was remarkably enhanced by a synergistic effect of crown ethers. A macrocyclic ligand (H
 DA18C6) composed of diaza-18-crown-6 and two 4-acyl-5-pyrazolones was synthesized for the extraction of Sr(II). The extraction performance of H
DA18C6) composed of diaza-18-crown-6 and two 4-acyl-5-pyrazolones was synthesized for the extraction of Sr(II). The extraction performance of H
 DA18C6 was significantly enhanced only in [C
DA18C6 was significantly enhanced only in [C mim][Tf
mim][Tf N] by the intramolecular cooperative effect.
N] by the intramolecular cooperative effect.
Sugita, Tsuyoshi; Fujiwara, Iori*; Okamura, Hiroyuki; Oshima, Tatsuya*; Baba, Yoshinari*; Naganawa, Hirochika; Shimojo, Kojiro
Solvent Extraction Research and Development, Japan, 24(2), p.61 - 69, 2017/05
We investigated an influence of amide group in diglycolamic acid-type extractants on extraction property of metal ions. The extraction characteristics of  -dodecyldiglycolamic acid (C
-dodecyldiglycolamic acid (C DGAA), with a secondary amide group, for 56 metal ions have been investigated, and compared with those of
DGAA), with a secondary amide group, for 56 metal ions have been investigated, and compared with those of  -dioctyldiglycolamic acid (DODGAA) with a tertiary amide group. Compared with DODGAA, C
-dioctyldiglycolamic acid (DODGAA) with a tertiary amide group. Compared with DODGAA, C DGAA has a poor extraction performance and separation ability for rare-earth metal ions, except for Sc(III). However, C
DGAA has a poor extraction performance and separation ability for rare-earth metal ions, except for Sc(III). However, C DGAA tended to provide better extraction for relatively small-sized metal ions than DODGAA. In addition, it was found that C
DGAA tended to provide better extraction for relatively small-sized metal ions than DODGAA. In addition, it was found that C DGAA enables the selective removal of Hg(II) from aqueous solutions containing various divalent metal ions.
DGAA enables the selective removal of Hg(II) from aqueous solutions containing various divalent metal ions.
 N
N anions in the ionic liquid-water distribution of europium(III) chelates
anions in the ionic liquid-water distribution of europium(III) chelatesOkamura, Hiroyuki; Aoyagi, Noboru; Shimojo, Kojiro; Naganawa, Hirochika; Imura, Hisanori*
RSC Advances (Internet), 7(13), p.7610 - 7618, 2017/01
Times Cited Count:20 Percentile:56.92(Chemistry, Multidisciplinary)The role of bis(trifluoromethanesulfonyl)imide (Tf N
N ) anions in the ionic liquid-water distribution systems of the Eu(III) chelates with 2-thenoyltrifluoroacetone (Htta) was investigated by the liquid-liquid distribution and time-resolved laser-induced fluorescence spectroscopy (TRLFS). The effect of the ionic liquids on the distribution constant of Eu(tta)
) anions in the ionic liquid-water distribution systems of the Eu(III) chelates with 2-thenoyltrifluoroacetone (Htta) was investigated by the liquid-liquid distribution and time-resolved laser-induced fluorescence spectroscopy (TRLFS). The effect of the ionic liquids on the distribution constant of Eu(tta) was evaluated by the regular solution theory. The distribution constant of Eu(tta)
was evaluated by the regular solution theory. The distribution constant of Eu(tta) in 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C
in 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C mim][Tf
mim][Tf N]) was increased dramatically by the solvation effects of Eu(tta)
N]) was increased dramatically by the solvation effects of Eu(tta) in [C
in [C mim][Tf
mim][Tf N]. TRLFS for [Eu(tta)
N]. TRLFS for [Eu(tta) (H
(H O)
O) ] synthesized revealed that the Eu(tta)
] synthesized revealed that the Eu(tta) chelate was almost completely dehydrated in a series of [C
chelate was almost completely dehydrated in a series of [C mim][Tf
mim][Tf N]. The Eu(tta)
N]. The Eu(tta) chelate exists as di- or tri-hydrates in 1-ethyl-3-methylimidazolium perchlorate ([C
chelate exists as di- or tri-hydrates in 1-ethyl-3-methylimidazolium perchlorate ([C mim][ClO
mim][ClO ]) containing 20 M water, whereas mono-hydrated chelate was formed in [C
]) containing 20 M water, whereas mono-hydrated chelate was formed in [C mim][Tf
mim][Tf N, ClO
N, ClO ] in the presence of 0.50 M Tf
] in the presence of 0.50 M Tf N
N and 20 M water. These results show that the coordinated water molecules of [Eu(tta)
and 20 M water. These results show that the coordinated water molecules of [Eu(tta) (H
(H O)
O) ] were replaced by the Tf
] were replaced by the Tf N
N anions. In fact, an anionic adduct, [Eu(tta)
anions. In fact, an anionic adduct, [Eu(tta) (Tf
(Tf N)]
N)] , was observed by electrospray ionization mass spectrometry in the presence of [C
, was observed by electrospray ionization mass spectrometry in the presence of [C mim][Tf
mim][Tf N].
N].
Okamura, Hiroyuki; Shimojo, Kojiro
Ion Ekitai Kenkyu Saizensen To Shakai Jisso, p.220 - 227, 2016/12
Solvent extraction is a separation method based on the difference in the distribution of solutes between two immiscible liquid phases. Recently, ionic liquids have been widely investigated as novel extraction media. Solvent properties of an ionic liquid can be adjusted by combination of a cationic and an anionic component. It is, therefore, possible to provide an attractive reaction field as an extraction medium. In this article, specific extraction phenomena observed in the ionic liquid extraction system for metal ions were introduced.
Sako, Hiroyuki; Harada, Hiroyuki; Sakaguchi, Takao*; Chujo, Tatsuya*; Esumi, Shinichi*; Gunji, Taku*; Hasegawa, Shoichi; Hwang, S.; Ichikawa, Yudai; Imai, Kenichi; et al.
Nuclear Physics A, 956, p.850 - 853, 2016/12
Times Cited Count:12 Percentile:65.66(Physics, Nuclear)Shimojo, Kojiro; Fujiwara, Iori*; Fujisawa, Kiyoshi*; Okamura, Hiroyuki; Sugita, Tsuyoshi; Oshima, Tatsuya*; Baba, Yoshinari*; Naganawa, Hirochika
Solvent Extraction Research and Development, Japan, 23(2), p.151 - 159, 2016/05
Liquid-liquid extraction of rare-earth (RE) cations has been investigated using  -dodecyldiglycolamic acid (C
-dodecyldiglycolamic acid (C DGAA) with a secondary amide group, and compared with that using
DGAA) with a secondary amide group, and compared with that using  -dioctyldiglycolamic acid (DODGAA) with a tertiary amide group. C
-dioctyldiglycolamic acid (DODGAA) with a tertiary amide group. C DGAA enables quantitative transfer of all RE cations from moderately acidic solution, while being selective toward the heavier RE cations, and performs better than typical carboxylic-acid-type extractants. However, C
DGAA enables quantitative transfer of all RE cations from moderately acidic solution, while being selective toward the heavier RE cations, and performs better than typical carboxylic-acid-type extractants. However, C DGAA provides low extraction performance and separation ability for RE cations compared with DODGAA because of the weaker basicity of the amide oxygen. Slope analysis demonstrated that RE
DGAA provides low extraction performance and separation ability for RE cations compared with DODGAA because of the weaker basicity of the amide oxygen. Slope analysis demonstrated that RE transfer with C
transfer with C DGAA proceeded through a proton-exchange reaction, forming a 1:3 complex, RE(C
DGAA proceeded through a proton-exchange reaction, forming a 1:3 complex, RE(C DGAA)
DGAA) . Structural characterization by X-ray diffraction revealed that three
. Structural characterization by X-ray diffraction revealed that three  -butyldiglycolamic acid (C
-butyldiglycolamic acid (C DGAA) molecules coordinated to the La
DGAA) molecules coordinated to the La central ion in a tridentate fashion and the La
central ion in a tridentate fashion and the La primary coordination sphere consisted of three oxygen atoms from the amide group, three oxygen atoms from the ether group, and three oxygen atoms from the carboxy group.
primary coordination sphere consisted of three oxygen atoms from the amide group, three oxygen atoms from the ether group, and three oxygen atoms from the carboxy group.
Naruto, Kenichi*; Nishino, Hiroyuki; Kurisaka, Kenichi; Yamano, Hidemasa; Okano, Yasushi; Okamura, Shigeki*; Eto, Masao*
Proceedings of 9th Korea-Japan Symposium on Nuclear Thermal Hydraulics and Safety (NTHAS-9) (CD-ROM), 10 Pages, 2014/11
 -diketone fragments on the extraction of strontium into an ionic liquid
-diketone fragments on the extraction of strontium into an ionic liquidOkamura, Hiroyuki; Naganawa, Hirochika; Imura, Hisanori*; Shimojo, Kojiro
Proceedings of 20th International Solvent Extraction Conference (ISEC 2014), p.1046 - 1051, 2014/09
 ,
, -dioctyldiglycolamic acid
-dioctyldiglycolamic acidShimojo, Kojiro; Nakai, Ayaka*; Okamura, Hiroyuki; Saito, Takumi*; Ohashi, Akira*; Naganawa, Hirochika
Analytical Sciences, 30(4), p.513 - 517, 2014/04
Times Cited Count:15 Percentile:48.57(Chemistry, Analytical)