Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Saito, Takumi*; Nishi, Shusaku*; Amano, Yuki; Beppu, Hikari*; Miyakawa, Kazuya
ACS ES&T Water (Internet), 3(12), p.4103 - 4112, 2023/12
Saito, Takumi*; Motokawa, Ryuhei; Okubo, Takahiro*; Miura, Daisuke*; Kumada, Takayuki
Environmental Science & Technology, 57(26), p.9802 - 9810, 2023/07
Times Cited Count:0 Percentile:0(Engineering, Environmental)Murota, Kento*; Aoyagi, Noboru; Mei, H.; Saito, Takumi*
Applied Geochemistry, 152, p.105620_1 - 105620_11, 2023/05
Times Cited Count:1 Percentile:62.05(Geochemistry & Geophysics)Hirata, Sakiko*; Kusaka, Ryoji; Meiji, Shogo*; Tamekuni, Seita*; Okudera, Kosuke*; Hamada, Shoken*; Sakamoto, Chihiro*; Honda, Takumi*; Matsushita, Kosuke*; Muramatsu, Satoru*; et al.
Inorganic Chemistry, 62(1), p.474 - 486, 2023/01
Times Cited Count:0 Percentile:0.01(Chemistry, Inorganic & Nuclear)Rizaal, M.; Nakajima, Kunihisa; Saito, Takumi*; Osaka, Masahiko; Okamoto, Koji*
ACS Omega (Internet), 7(33), p.29326 - 29336, 2022/08
Times Cited Count:2 Percentile:29.84(Chemistry, Multidisciplinary)Tachi, Yukio; Saito, Takumi*; Kirishima, Akira*
Nihon Genshiryoku Gakkai-Shi ATOMO , 64(5), p.290 - 295, 2022/05
, 64(5), p.290 - 295, 2022/05
no abstracts in English
 C control rods in Fukushima Daiichi nuclear reactors during meltdown; B-Li isotopic signatures in cesium-rich microparticles
C control rods in Fukushima Daiichi nuclear reactors during meltdown; B-Li isotopic signatures in cesium-rich microparticlesFueda, Kazuki*; Takami, Ryu*; Minomo, Kenta*; Morooka, Kazuya*; Horie, Kenji*; Takehara, Mami*; Yamasaki, Shinya*; Saito, Takumi*; Shiotsu, Hiroyuki; Onuki, Toshihiko*; et al.
Journal of Hazardous Materials, 428, p.128214_1 - 128214_10, 2022/04
Times Cited Count:6 Percentile:68.71(Engineering, Environmental) ion complex; Presence of a Rh
ion complex; Presence of a Rh Intermediate in Direct Photoreduction
Intermediate in Direct PhotoreductionSaeki, Morihisa*; Matsumura, Daiju; Nakanishi, Ryuzo*; Yomogida, Takumi; Tsuji, Takuya; Saito, Hiroyuki*; Oba, Hironori*
Journal of Physical Chemistry C, 126(12), p.5607 - 5616, 2022/03
Times Cited Count:1 Percentile:14.66(Chemistry, Physical)The reaction mechanism of the direct photoreduction of a Rh ion complex to a Rh
ion complex to a Rh species induced by pulsed ultraviolet laser irradiation was studied using dispersive X-ray absorption fine structure (DXAFS) spectroscopy. The time-resolved X-ray absorption near edge structure (XANES) showed the absence of isosbestic points and suggested that more than two Rh
species induced by pulsed ultraviolet laser irradiation was studied using dispersive X-ray absorption fine structure (DXAFS) spectroscopy. The time-resolved X-ray absorption near edge structure (XANES) showed the absence of isosbestic points and suggested that more than two Rh species contribute toward the direct photoreduction of Rh
species contribute toward the direct photoreduction of Rh . Analysis of the time-resolved XANES data by singular value deposition showed that the direct photoreduction involves three Rh
. Analysis of the time-resolved XANES data by singular value deposition showed that the direct photoreduction involves three Rh species. Multivariate curve resolution by alternating least-squares analysis (MCR-ALS) of the time-resolved XANES data gave pure spectra and concentration profiles of the three Rh
species. Multivariate curve resolution by alternating least-squares analysis (MCR-ALS) of the time-resolved XANES data gave pure spectra and concentration profiles of the three Rh species. The Rh
species. The Rh species were assigned to Rh
species were assigned to Rh , Rh
, Rh , and Rh
, and Rh species based on the features of the pure XANES spectra. The concentration profiles suggested that the direct photoreduction proceeds in the order of Rh
species based on the features of the pure XANES spectra. The concentration profiles suggested that the direct photoreduction proceeds in the order of Rh
 Rh
Rh
 Rh
Rh . A reaction mechanism, which was proposed involving photoreductions of Rh
. A reaction mechanism, which was proposed involving photoreductions of Rh and Rh
and Rh , photoinduced autocatalytic reductions of Rh
, photoinduced autocatalytic reductions of Rh and Rh
and Rh , and photooxidation of Rh
, and photooxidation of Rh , well reproduced the concentration profiles of three Rh
, well reproduced the concentration profiles of three Rh species.
species.
Mei, H.; Aoyagi, Noboru; Saito, Takumi*; Kozai, Naofumi; Sugiura, Yuki; Tachi, Yukio
Applied Geochemistry, 136, p.105178_1 - 105178_8, 2022/01
Times Cited Count:11 Percentile:86.47(Geochemistry & Geophysics) and Tb
and Tb measured by gel electrophoresis techniques
measured by gel electrophoresis techniquesNakano, Sumika*; Marumo, Kazuki*; Kazami, Rintaro*; Saito, Takumi*; Haraga, Tomoko; Tasaki-Handa, Yuiko*; Saito, Shingo*
Environmental Science & Technology, 55(22), p.15172 - 15180, 2021/11
Times Cited Count:5 Percentile:35.21(Engineering, Environmental)Humic acid (HA) can strongly complex with metal ions to form a supramolecular assembly via coordination binding. However, determining the supramolecular size distribution and stoichiometry between small HA unit molecules constituting HA supramolecule and metal ions has proven to be challenging. Here, we investigated the changes in the size distributions of HAs induced by Cu and Tb
and Tb ions using a unique polyacrylamide gel electrophoresis (PAGE) for the separation and quantification of HA complexes and metal ions bound, followed by UV-Vis spectroscopy and EEM-PARAFAC. It was found that the supramolecular behaviors of Cu
ions using a unique polyacrylamide gel electrophoresis (PAGE) for the separation and quantification of HA complexes and metal ions bound, followed by UV-Vis spectroscopy and EEM-PARAFAC. It was found that the supramolecular behaviors of Cu and Tb
and Tb complexes with HA collected from peat and deep groundwater (HHA) differed. Our results suggest that this supramolecular stoichiometry is related to the abundance of sulfur atoms in the elemental composition of HHA. Our results provide new insights into HA supramolecules formed via metal complexation.
complexes with HA collected from peat and deep groundwater (HHA) differed. Our results suggest that this supramolecular stoichiometry is related to the abundance of sulfur atoms in the elemental composition of HHA. Our results provide new insights into HA supramolecules formed via metal complexation.
Zhou, Q.*; Saito, Takumi*; Suzuki, Seiya; Yano, Kimihiko; Suzuki, Shunichi*
Journal of Nuclear Science and Technology, 58(4), p.461 - 472, 2021/04
Times Cited Count:6 Percentile:65.59(Nuclear Science & Technology)Saeki, Morihisa*; Yomogida, Takumi; Matsumura, Daiju; Saito, Takumi*; Nakanishi, Ryuzo*; Tsuji, Takuya; Oba, Hironori*
Analytical Sciences, 36(11), p.1371 - 1378, 2020/11
Times Cited Count:2 Percentile:10(Chemistry, Analytical)We measured X-ray absorption fine structure (XAFS) and Raman spectra of isopolymolybdates(VI) in HNO solution (0.15- 4.0 M), which change their geometries depending on acid concentration, and performed simultaneous resolution of the XAFS and Raman data using a multivariate curve resolution by alternating least-squares (MCR-ALS) analysis. In iterative ALS optimization, initial data matrices were prepared by two different methods. The MCR-ALS result of single XAFS data matrix shows large dependence on the preparation method of the initial data matrices. The MCR-ALS result of an augmented matrix of Raman and XAFS data has little dependence on the initial data matrices. It indicates that the augmentation method effectively improves the rotation ambiguities in the MCR-ALS analysis of the XAFS data. Based on the model fitting of the pure EXAFS oscillations, we revealed the change of [Mo
solution (0.15- 4.0 M), which change their geometries depending on acid concentration, and performed simultaneous resolution of the XAFS and Raman data using a multivariate curve resolution by alternating least-squares (MCR-ALS) analysis. In iterative ALS optimization, initial data matrices were prepared by two different methods. The MCR-ALS result of single XAFS data matrix shows large dependence on the preparation method of the initial data matrices. The MCR-ALS result of an augmented matrix of Raman and XAFS data has little dependence on the initial data matrices. It indicates that the augmentation method effectively improves the rotation ambiguities in the MCR-ALS analysis of the XAFS data. Based on the model fitting of the pure EXAFS oscillations, we revealed the change of [Mo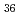 O
O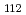 (H
(H O)
O)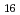 ]
]
 [Mo
[Mo O
O (H
(H O)
O) ]
]
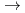 [HMoO
[HMoO (H
(H O)
O) ]
] in the highly concentrated HNO
in the highly concentrated HNO solution.
solution.
Rizaal, M.; Nakajima, Kunihisa; Saito, Takumi*; Osaka, Masahiko; Okamoto, Koji*
Journal of Nuclear Science and Technology, 57(9), p.1062 - 1073, 2020/09
Times Cited Count:8 Percentile:71.58(Nuclear Science & Technology)The interaction of cesium hydroxide and a calcium silicate insulation material was experimentally investigated at high temperature conditions. A thermogravimetry equipped with differential thermal analysis was used to analyze thermal events in the samples of mixed calcium silicate and cesium hydroxide under Ar-5%H and Ar-4%H
and Ar-4%H -20%H
-20%H 0 with maximum temperature of 1100
0 with maximum temperature of 1100 C. Prior being mixed with cesium hydroxide, a part of calcium silicate was pretreated at high temperature to evaluate the effect of possible structural changes of this material due to a preceding thermal history and also the sake of thermodynamic evaluation to those available ones. Based upon the initial condition (preliminary heat treatment) of calcium silicate, it was found that if the original material consisted of xonotlite (Ca
C. Prior being mixed with cesium hydroxide, a part of calcium silicate was pretreated at high temperature to evaluate the effect of possible structural changes of this material due to a preceding thermal history and also the sake of thermodynamic evaluation to those available ones. Based upon the initial condition (preliminary heat treatment) of calcium silicate, it was found that if the original material consisted of xonotlite (Ca Si
Si 0
0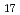 (0H)
(0H) ), the endothermic reaction with cesium hydroxide occurred over the temperature range 575-730
), the endothermic reaction with cesium hydroxide occurred over the temperature range 575-730 C meanwhile if the crystal phase of original material was changed to wollastonite (CaSi0
C meanwhile if the crystal phase of original material was changed to wollastonite (CaSi0 ), the interaction occurred over temperature range 700-1100
), the interaction occurred over temperature range 700-1100 C. Furthermore, the X-ray diffraction analyses have indicated on both type of pretreated calsils that regardless of Ar-5%H
C. Furthermore, the X-ray diffraction analyses have indicated on both type of pretreated calsils that regardless of Ar-5%H and Ar-4%H
and Ar-4%H -20%H
-20%H 0 atmosphere, cesium aluminum silicate, CsAlSi0
0 atmosphere, cesium aluminum silicate, CsAlSi0 was formed with aluminum in the samples as an impurity or adduct.
was formed with aluminum in the samples as an impurity or adduct.
 Se
Se ultrathin films on van der Waals ferromagnet Cr
ultrathin films on van der Waals ferromagnet Cr Si
Si Te
Te
Kato, Takemi*; Sugawara, Katsuaki*; Ito, Naohiro*; Yamauchi, Kunihiko*; Sato, Takumi*; Oguchi, Tamio*; Takahashi, Takashi*; Shiomi, Yuki*; Saito, Eiji; Sato, Takafumi*
Physical Review Materials (Internet), 4(8), p.084202_1 - 084202_6, 2020/08
Times Cited Count:4 Percentile:20.66(Materials Science, Multidisciplinary)Rizaal, M.; Saito, Takumi*; Okamoto, Koji*; Erkan, N.*; Nakajima, Kunihisa; Osaka, Masahiko
Mechanical Engineering Journal (Internet), 7(3), p.19-00563_1 - 19-00563_10, 2020/06
The adsorption of cesium (Cs) on calcium silicate insulation of primary piping system is postulated to contribute in high dose rate of surrounding pedestal area in Fukushima Daiichi NPP unit 2. In this study, room-temperature experiment of Cs adsorption on calcium silicate has been studied as an initial approach of Cs adsorption behavior toward higher temperature condition. As the result of analyzing of Cs adsorption kinetics, it was expected that the underlying adsorption mechanism is chemisorption. Furthermore, analysis of adsorption isotherm suggested unrestricted monolayer formation followed by multilayer formation.
 -humate complexes
-humate complexesMarumo, Kazuki*; Matsumoto, Atsumasa*; Nakano, Sumika*; Shibukawa, Masami*; Saito, Takumi*; Haraga, Tomoko; Saito, Shingo*
Environmental Science & Technology, 53(24), p.14507 - 14515, 2019/12
Times Cited Count:7 Percentile:29.01(Engineering, Environmental)Humic acids (HA) are responsible for the fate of metal ions in the environment. We developed a polyacrylamide gel electrophoresis (PAGE) technique to investigate the MW distributions of metal ion (copper ion). Combining contaminant-metal-free and high-resolution PAGE systems for HA provided accurate MW distributions for the metal ions. Coupling this system with UV-Vis spectrometry and the excitation-emission matrix (EEM) spectrometry-parallel factor analysis (PARAFAC) method revealed new insights into metal-HA complex. Interestingly, the MW distributions of the three metal ions were entirely different, indicating that the presence of specific binding environments in HA for the metal ions depending its MW. The MW distributions of five fluorescent components were associated with the metal ion distributions. Our PAGE-based methodology suggests that metal binding sites and fluorescent components in HA exhibit heterogeneity in terms of metal binding affinity and MW.
 , Zn
, Zn +, and Co
+, and Co
Kato, Tomoaki*; Yu, Q.*; Tanaka, Kazuya; Kozai, Naofumi; Saito, Takumi*; Onuki, Toshihiko
Journal of Environmental Sciences, 86, p.78 - 86, 2019/12
Times Cited Count:2 Percentile:8.42(Environmental Sciences)This paper investigated the fate of the dissolved permanganate in aqueous solution after contact with bacterial cells and metal accumulation during precipitation of Mn oxides. When Mn(VII) was contacted with bacterial cells, cells were damaged and Mn(VII) was reduced by cells to lower valence and precipitated as Mn oxides (biomass Mn oxides). When Co ions were present, Co was incorporated into Mn oxides as Co
ions were present, Co was incorporated into Mn oxides as Co . These results suggest that Mn(VII) can be used to remove metal ions when introduced to wastewater as disinfectant.
. These results suggest that Mn(VII) can be used to remove metal ions when introduced to wastewater as disinfectant.
Kobayashi, Taishi*; Nakajima, Shogo*; Motokawa, Ryuhei; Matsumura, Daiju; Saito, Takumi*; Sasaki, Takayuki*
Langmuir, 35(24), p.7995 - 8006, 2019/06
Times Cited Count:5 Percentile:22.5(Chemistry, Multidisciplinary)Strasser, P.*; Abe, Mitsushi*; Aoki, Masaharu*; Choi, S.*; Fukao, Yoshinori*; Higashi, Yoshitaka*; Higuchi, Takashi*; Iinuma, Hiromi*; Ikedo, Yutaka*; Ishida, Katsuhiko*; et al.
EPJ Web of Conferences, 198, p.00003_1 - 00003_8, 2019/01
Times Cited Count:13 Percentile:99.06(Quantum Science & Technology)Saeki, Morihisa*; Matsumura, Daiju; Yomogida, Takumi; Taguchi, Tomitsugu*; Tsuji, Takuya; Saito, Hiroyuki*; Oba, Hironori*
Journal of Physical Chemistry C, 123(1), p.817 - 824, 2019/01
Times Cited Count:14 Percentile:53.94(Chemistry, Physical)Reaction kinetics of laser-induced particle formation in an aqueous solution of PdCl
 was investigated by transmission electron microscope (TEM) and dispersive X-ray absorption fine structure (DXAFS). The Pd particle was generated by irradiation of nanosecond pulsed 266-nm laser. The TEM observation showed dependence of the particle size on the laser fluence and promotion of the particle growth by irradiation of high-fluence laser. The DXAFS data give us the Pd
was investigated by transmission electron microscope (TEM) and dispersive X-ray absorption fine structure (DXAFS). The Pd particle was generated by irradiation of nanosecond pulsed 266-nm laser. The TEM observation showed dependence of the particle size on the laser fluence and promotion of the particle growth by irradiation of high-fluence laser. The DXAFS data give us the Pd concentration. Temporal changes of the Pd
concentration. Temporal changes of the Pd concentration analyzed based on Finke-Watzky two step mechanism. The analysis elucidates that the laser photon contributes to the reduction of the PdCl
concentration analyzed based on Finke-Watzky two step mechanism. The analysis elucidates that the laser photon contributes to the reduction of the PdCl
 ion by the one-photon process and to the autocatalytic growth of the Pd particles by the multi-photon process.
ion by the one-photon process and to the autocatalytic growth of the Pd particles by the multi-photon process.