Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Abeykoon, S.*; Howard, C.*; Dominijanni, S.*; Eberhard, L.*; Kurnosov, A.*; Frost, D. J.*; Boffa Ballaran, T.*; Terasaki, Hidenori*; Sakamaki, Tatsuya*; Suzuki, Akio*; et al.
Journal of Geophysical Research; Solid Earth, 128(9), p.e2023JB026710_1 - e2023JB026710_17, 2023/09
Times Cited Count:0 Percentile:0.01(Geochemistry & Geophysics)Small amounts of iron sulphide minerals are found in most rocks from the Earth's mantle and as inclusions trapped in natural diamonds. Hydrogen may dissolve into iron sulphide minerals under high pressures and temperature, but is most likely lost once pressure and temperature are removed. In this study, we determined deuterium contents in iron sulphide, held under high pressure and temperature conditions, using neutron diffraction measurements with 6-ram multi-anvil press at PLANET, J-PARC. Deuterium contents in iron sulphide were measured at high-P, up to 11.4 GPa and high-T to 1300 K in in situ neutron diffraction experiments. The total deuterium content increases with both P and T. The results are used to estimate hydrogen contents of iron sulphide minerals in the deep continental lithospheric mantle, which are found to be in the range 1700-2700 ppm. This corresponds to approximately 2-3 ppm of hydrogen in the bulk mantle.
Ohashi, Tomonori*; Sakamaki, Tatsuya*; Funakoshi, Kenichi*; Hattori, Takanori; Hisano, Naoki*; Abe, Jun*; Suzuki, Akio*
American Mineralogist, 107(3), p.325 - 335, 2022/03
Times Cited Count:1 Percentile:22.72(Geochemistry & Geophysics)The basaltic glass structure were investigated to 18 GPa using in situ X-ray and neutron diffraction. The O-O coordination number (CN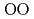 ) starts to rise with maintaining the mean O-O distance (r
) starts to rise with maintaining the mean O-O distance (r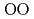 ) above 2-4 GPa, and then CN
) above 2-4 GPa, and then CN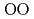 stops increasing and r
stops increasing and r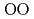 begins to shrink along with the increase in the Al-O coordination number (CN
begins to shrink along with the increase in the Al-O coordination number (CN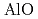 ) above 9 GPa. This is interpreted by the change in the contraction mechanism from tetrahedral network bending to oxygen packing ratio increase via the CN
) above 9 GPa. This is interpreted by the change in the contraction mechanism from tetrahedral network bending to oxygen packing ratio increase via the CN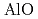 increase. The oxygen packing fraction exceeds the value for dense random packing, suggesting that the oxygen-packing hypothesis cannot account for the pressure-induced structural transformations of silica and silicate glasses. The CN
increase. The oxygen packing fraction exceeds the value for dense random packing, suggesting that the oxygen-packing hypothesis cannot account for the pressure-induced structural transformations of silica and silicate glasses. The CN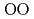 increase at 2-4 GPa reflects the elastic softening of silicate glass, which may causes anomalous elastic moduli of basaltic glass at
increase at 2-4 GPa reflects the elastic softening of silicate glass, which may causes anomalous elastic moduli of basaltic glass at  2 GPa.
2 GPa.
 O in the film on Fe oxidized at high temperature in air
O in the film on Fe oxidized at high temperature in airHaruna, Takumi*; Yamamoto, Tatsuya*; Miyairi, Yoji*; Shibata, Toshio*; Taniguchi, Naoki; Sakamaki, Keiko; Tachikawa, Hirokazu*
Zairyo To Kankyo, 64(5), p.201 - 206, 2015/05
Diffusion coefficients of D O in the films was determined in order to estimate corrosion rate of carbon steel for the overpack in ground water. Fe plates were heated to form oxide films. The films were characterized with XRD and SEM. After that, the specimen was contacted with D
O in the films was determined in order to estimate corrosion rate of carbon steel for the overpack in ground water. Fe plates were heated to form oxide films. The films were characterized with XRD and SEM. After that, the specimen was contacted with D O for 5184 ks, followed by subjected to TDS to obtain an amount of D
O for 5184 ks, followed by subjected to TDS to obtain an amount of D O absorbing into the film. As a result, single-layered film of Fe
O absorbing into the film. As a result, single-layered film of Fe O
O was formed at 573 and 723 K, and double-layered film of Fe
was formed at 573 and 723 K, and double-layered film of Fe O
O and Fe
and Fe O
O was formed at 873 K. It was found that an amount of D
was formed at 873 K. It was found that an amount of D O in the film correlated linearly with a square root of the absorption period, and that the amount was steady for a long period. From the results and Fick's second law, diffusion coefficients of D
O in the film correlated linearly with a square root of the absorption period, and that the amount was steady for a long period. From the results and Fick's second law, diffusion coefficients of D O was determined as 9.7
O was determined as 9.7 10
10 cm
cm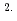 s
s for the Fe
for the Fe O
O film, and 5.5
film, and 5.5 10
10 cm
cm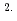 s
s to 2.2
to 2.2 10
10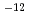 cm
cm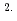 s
s for Fe
for Fe O
O film.
film.
Sakamaki, Tatsuya*; Suzuki, Akio*; Otani, Eiji*; Terasaki, Hidenori*; Urakawa, Satoru*; Katayama, Yoshinori; Funakoshi, Kenichi*; Wang, Y.*; Hernlund, J. W.*; Ballmer, M. D.*
Nature Geoscience, 6(12), p.1041 - 1044, 2013/12
Times Cited Count:128 Percentile:96.48(Geosciences, Multidisciplinary)The bounday between Earth's rigid lighosphere and the underlying, ductile ashenosphere is marked by a distinct siseismic discontinuity. We measure the density, viscosity and structure of basaltic magmas using high-pressure and high-temperature experiments and in situ X-ray analysis under pressure of up to 5.5 GPa. We find that the magmas rapidly become denser with increasing presure and show a viscosity minimum near 4 GPa. Magma mobility determined by the density and viscosity data exhibits a peak at pressures corresponding to depths of 120-150 km, within the asthenosphere. The diminishing mobility of magma in Earth's asthenosphere as the mlets ascend could lead to excessive melt accumulation at depths of 80-100 km, at the lithosphere-asthenosphere boundary. It is concluded that the observed seismic discontinuity at the lithosphere-asthenosphere boundary records this accumulation of melt.
Nishida, Keisuke*; Otani, Eiji*; Urakawa, Satoru*; Suzuki, Akio*; Sakamaki, Tatsuya*; Terasaki, Hidenori*; Katayama, Yoshinori
American Mineralogist, 96(5-6), p.864 - 868, 2011/05
Times Cited Count:32 Percentile:69.49(Geochemistry & Geophysics)The density of liquid iron sulfide (FeS) was measured up to 3.8 GPa and 1800 K using the X-ray absorption method. The compression curve of the liquid FeS can be fitted using the Vinet equation of state. Isothermal bulk modulus and its temperature and pressure derivatives were determined by a non-linear least squares fit. Liquid FeS is more compressible than Fe-rich Fe-S liquid.
Sakamaki, Tatsuya*; Otani, Eiji*; Urakawa, Satoru*; Terasaki, Hidenori*; Katayama, Yoshinori
American Mineralogist, 96(4), p.553 - 557, 2011/04
Times Cited Count:31 Percentile:68.33(Geochemistry & Geophysics)The density of carbonated peridotite magma was measured up to 3.8 GPa and 2100 K using an X-ray absorption method. The bulk modulus of carbonated peridotite magma is larger than that of hydrous peridotite magma. The partial molar volume of CO in magma under high pressure and temperature conditions was calculated. Our results show that the partial molar volume of CO
in magma under high pressure and temperature conditions was calculated. Our results show that the partial molar volume of CO is less compressible than that of H
is less compressible than that of H O, suggesting that, on an equal molar basis, CO
O, suggesting that, on an equal molar basis, CO is more effective than H
is more effective than H O in reducing peridotite melt density at high pressure.
O in reducing peridotite melt density at high pressure.
Sakamaki, Tatsuya*; Otani, Eiji*; Urakawa, Satoru*; Suzuki, Akio*; Katayama, Yoshinori; Zhao, D.*
Earth and Planetary Science Letters, 299(3-4), p.285 - 289, 2010/11
Times Cited Count:29 Percentile:58.17(Geochemistry & Geophysics)The density of the Apollo 14 black glass melt, which has the highest TiO content of pristine mare glasses, was measured to 4.8 GPa and 2100 K using an X-ray absorption method. A fit of the pressure-density-temperature data to the high-temperature Birch-Murnaghan equation of state yielded the isothermal bulk modulus, its pressure derivative, and the temperature derivative of the bulk modulus. Implication for heterogeneities in the lunar mantle is discussed.
content of pristine mare glasses, was measured to 4.8 GPa and 2100 K using an X-ray absorption method. A fit of the pressure-density-temperature data to the high-temperature Birch-Murnaghan equation of state yielded the isothermal bulk modulus, its pressure derivative, and the temperature derivative of the bulk modulus. Implication for heterogeneities in the lunar mantle is discussed.
Sakamaki, Tatsuya*; Otani, Eiji*; Urakawa, Satoru*; Suzuki, Akio*; Katayama, Yoshinori
American Mineralogist, 95(1), p.144 - 147, 2010/01
Times Cited Count:37 Percentile:70.43(Geochemistry & Geophysics)The density of a peridotite magma was measured up to 2.5 GPa and 2300 K using an X-ray absorption method. The method allowed measurement of the density of a peridotite melt under seven different conditions and clarified the pressure and temperature dependence of the density. A fit of the pressure-density-temperature data to the high-temperature Birch-Murnaghan equation of state yielded the isothermal bulk modulus, 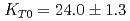 GPa, its pressure derivative,
GPa, its pressure derivative, 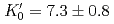 , and the derivative of bulk modulus
, and the derivative of bulk modulus  GPa/K at 2100 K. The large bulk modulus and its pressure derivative of the peridotite melt compared with that of basaltic melt is consistent with previous results from sink-float experiments.
GPa/K at 2100 K. The large bulk modulus and its pressure derivative of the peridotite melt compared with that of basaltic melt is consistent with previous results from sink-float experiments.
Sakamaki, Tatsuya*; Otani, Eiji*; Urakawa, Satoru*; Suzuki, Akio*; Katayama, Yoshinori
Earth and Planetary Science Letters, 287(3-4), p.293 - 297, 2009/10
Times Cited Count:57 Percentile:78.64(Geochemistry & Geophysics)The density of hydrous peridotite magma containing 5wt.% H O was measured at pressures and temperatures up to 4.3 GPa and 2073 K, respectively, using the X-ray absorption method. A fit of pressure-density-temperature data to the high-temperature Birch-Murnagan equation of state yields isothermal bulk modulus
O was measured at pressures and temperatures up to 4.3 GPa and 2073 K, respectively, using the X-ray absorption method. A fit of pressure-density-temperature data to the high-temperature Birch-Murnagan equation of state yields isothermal bulk modulus 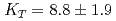 GPa, its pressure derivative
GPa, its pressure derivative 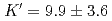 and the temperature derivative of the bulk modulus
and the temperature derivative of the bulk modulus 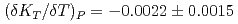 GPa/K at 1773K. The small bulk modulus of the hydrous peridotite magma compared with that of the dry peridotite magma reflects the effect of water, which is more compressible than the silicate melt.
GPa/K at 1773K. The small bulk modulus of the hydrous peridotite magma compared with that of the dry peridotite magma reflects the effect of water, which is more compressible than the silicate melt.