Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...

Chiera, N. M.*; Sato, Tetsuya; Eichler, R.*; Tomitsuka, Tomohiro; Asai, Masato; Adachi, Sadia*; Dressler, R.*; Hirose, Kentaro; Inoue, Hiroki*; Ito, Yuta; et al.
Angewandte Chemie; International Edition, 60(33), p.17871 - 17874, 2021/08
Times Cited Count:2 Percentile:14.88(Chemistry, Multidisciplinary)The formation and the chemical characterization of single atoms of dubnium (Db, element 105), in the form of its volatile oxychloride, was investigated using the on-line gas phase chromatography technique, in the temperature range 350 - 600 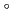 C. Under the exact same chemical conditions, comparative studies with the lighter homologs of group-5 in the Periodic Table clearly indicate the volatility sequence being NbOCl
C. Under the exact same chemical conditions, comparative studies with the lighter homologs of group-5 in the Periodic Table clearly indicate the volatility sequence being NbOCl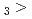 TaOCl
TaOCl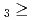 DbOCl
DbOCl . From the obtained experimental results, thermochemical data for DbOCl
. From the obtained experimental results, thermochemical data for DbOCl were derived. The present study delivers reliable experimental information for theoretical calculations on the chemical properties of transactinides.
were derived. The present study delivers reliable experimental information for theoretical calculations on the chemical properties of transactinides.
Chiera, N. M.; Sato, Tetsuya; Tomitsuka, Tomohiro; Asai, Masato; Ito, Yuta; Shirai, Kaori*; Suzuki, Hayato; Tokoi, Katsuyuki; Toyoshima, Atsushi; Tsukada, Kazuaki; et al.
Journal of Radioanalytical and Nuclear Chemistry, 320(3), p.633 - 642, 2019/06
Times Cited Count:1 Percentile:11.15(Chemistry, Analytical)An isothermal gas-chromatographic (IGC) device has been developed and tested for on-line gas phase studies of volatile oxychlorides of short-lived group-5 transition metals. Radioisotopes of niobium and tantalum, produced in nuclear fusion evaporation reactions, are directly flushed into the IGC setup by an inert gas-jet. Oxychloride compounds are formed by the addition of SOCl and O
and O . Parameters influencing the formation and transport of NbOCl
. Parameters influencing the formation and transport of NbOCl and TaOCl
and TaOCl are investigated. For nuclides with half-lives (
are investigated. For nuclides with half-lives (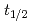 ) of about 30 s, an overall efficiency of 7% is obtained, rendering the IGC setup suitable for the chemical exploration of
) of about 30 s, an overall efficiency of 7% is obtained, rendering the IGC setup suitable for the chemical exploration of 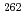 Db(
Db(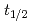 = 34s).
= 34s).
Sato, Tetsuya; Kaneya, Yusuke*; Asai, Masato; Tsukada, Kazuaki; Toyoshima, Atsushi; Mitsukai, Akina*; Osa, Akihiko; Makii, Hiroyuki; Nishio, Katsuhisa; Hirose, Kentaro; et al.
no journal, ,
The ground state electronic configuration of lawrencium (Lr, Z =103) is predicted to be [Rn]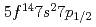 , which is different from that of the lanthanide homolog Lu [Xe]
, which is different from that of the lanthanide homolog Lu [Xe]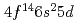 due to strong relativistic effects. According to semi-empirical considerations, volatility of Lr is expected to be higher than that of Lu. We have investigated adsorption behavior of
due to strong relativistic effects. According to semi-empirical considerations, volatility of Lr is expected to be higher than that of Lu. We have investigated adsorption behavior of 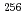 Lr, which was produced in the reaction of
Lr, which was produced in the reaction of 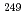 Cf(
Cf(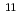 B, 4n), on a tantalum (Ta) metal surface using a surface ion-source installed into the isotope separator on-line (ISOL) at the JAEA tandem accelerator facility. The observed adsorption behavior of
B, 4n), on a tantalum (Ta) metal surface using a surface ion-source installed into the isotope separator on-line (ISOL) at the JAEA tandem accelerator facility. The observed adsorption behavior of 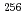 Lr was similar to those of Tb and Lu which have relatively higher adsorption enthalpy on Ta surface. It implies that Lr would have low volatility like such as Lu and Tb.
Lr was similar to those of Tb and Lu which have relatively higher adsorption enthalpy on Ta surface. It implies that Lr would have low volatility like such as Lu and Tb.
Sato, Tetsuya; Kaneya, Yusuke*; Asai, Masato; Tsukada, Kazuaki; Toyoshima, Atsushi; Mitsukai, Akina*; Osa, Akihiko; Makii, Hiroyuki; Hirose, Kentaro; Nagame, Yuichiro; et al.
no journal, ,
Our experimental results on the first ionization potential measurement of lawrencium (Lr, element 103) have strongly suggested that the Lr atom has a [Rn]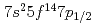 configuration as a result of the influence of strong relativistic effects. The configuration is different from that expected from the lanthanide homologue, lutetium (Lu). According to a semi-empirical consideration, it is expected that the change of the electronic configuration leads higher volatility of Lr than that of Lu. In this work, adsorption behaviors of Lr and various short-lived rare earth isotopes on a tantalum surface were investigated via observation of their surface ionization efficiencies. It was found that Lr would behave like low volatile rare earth elements such as Lu contrary to the semi-empirical expectation.
configuration as a result of the influence of strong relativistic effects. The configuration is different from that expected from the lanthanide homologue, lutetium (Lu). According to a semi-empirical consideration, it is expected that the change of the electronic configuration leads higher volatility of Lr than that of Lu. In this work, adsorption behaviors of Lr and various short-lived rare earth isotopes on a tantalum surface were investigated via observation of their surface ionization efficiencies. It was found that Lr would behave like low volatile rare earth elements such as Lu contrary to the semi-empirical expectation.
Kaneya, Yusuke*; Tomitsuka, Tomohiro; Sato, Tetsuya; Asai, Masato; Tsukada, Kazuaki; Toyoshima, Atsushi; Mitsukai, Akina; Makii, Hiroyuki; Hirose, Kentaro; Osa, Akihiko; et al.
no journal, ,
Kaneya, Yusuke*; Asai, Masato; Sato, Tetsuya; Tomitsuka, Tomohiro; Tsukada, Kazuaki; Toyoshima, Atsushi; Mitsukai, Akina; Makii, Hiroyuki; Hirose, Kentaro; Osa, Akihiko; et al.
no journal, ,
To study the influence of the valence 7p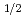 electronic orbital on chemical properties of lawrencium, a measurement of the adsorption enthalpy of lawrencium was carried out. A new method using a surface ionization technique coupled to an on-line isotope separator was developed, which enabled one to measure temperature dependence of lawrencium surface adsorption on a metallic tantalum surface at high temperature up to 2800 K. The temperature dependences of adsorption of lawrencium as well as various lanthanide elements were investigated with this method, and the adsorption enthalpy of lawrencium was successfully extracted.
electronic orbital on chemical properties of lawrencium, a measurement of the adsorption enthalpy of lawrencium was carried out. A new method using a surface ionization technique coupled to an on-line isotope separator was developed, which enabled one to measure temperature dependence of lawrencium surface adsorption on a metallic tantalum surface at high temperature up to 2800 K. The temperature dependences of adsorption of lawrencium as well as various lanthanide elements were investigated with this method, and the adsorption enthalpy of lawrencium was successfully extracted.
Sato, Tetsuya; Kaneya, Yusuke*; Asai, Masato; Tsukada, Kazuaki; Toyoshima, Atsushi; Mitsukai, Akina*; Osa, Akihiko; Makii, Hiroyuki; Hirose, Kentaro; Nagame, Yuichiro; et al.
no journal, ,
Our experimental results on the first ionization potential measurement of lawrencium (Lr, element 103) have strongly suggested that the Lr atom has a [Rn]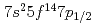 configuration as a result of the influence of strong relativistic effects. The configuration is different from that expected from the lanthanide homologue, lutetium (Lu). According to a semi-empirical consideration, it is expected that the change of the electronic configuration leads higher volatility of Lr than that of Lu. In this work, adsorption behaviors of Lr and various short-lived rare earth isotopes on a tantalum surface were investigated via observation of their surface ionization efficiencies. It was found that Lr would behave like low volatile rare earth elements such as Lu contrary to the semi-empirical expectation.
configuration as a result of the influence of strong relativistic effects. The configuration is different from that expected from the lanthanide homologue, lutetium (Lu). According to a semi-empirical consideration, it is expected that the change of the electronic configuration leads higher volatility of Lr than that of Lu. In this work, adsorption behaviors of Lr and various short-lived rare earth isotopes on a tantalum surface were investigated via observation of their surface ionization efficiencies. It was found that Lr would behave like low volatile rare earth elements such as Lu contrary to the semi-empirical expectation.
Sato, Tetsuya; Chiera, N. M.*; Tomitsuka, Tomohiro; Tokoi, Katsuyuki*; Suzuki, Hayato*; Ito, Yuta; Asai, Masato; Shirai, Kaori*; Inoue, Hiroki*; Adachi, Sadia*; et al.
no journal, ,
The influence of strong relativistic effects on chemical properties has been interesting in the superheavy element region. Their chemical properties, however, have not been investigated sufficiently because of experimental difficulties owing to their low production rates and short half-lives. In order to elucidate the chemical properties of dubnium (Db, Z = 105), we have conducted on-line isothermal gas chromatographic experiments of oxychloride of group-5 elements. We confirmed the formation of volatile oxychlorides of Db and its lighter homologs Nb and Ta by using 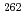 Db (half-life,
Db (half-life, 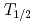 = 33.8 s),
= 33.8 s), 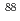 Nb (
Nb (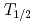 = 14.5 min.), and
= 14.5 min.), and 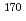 Ta (
Ta (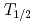 = 6.76 min.), respectively. We successfully determined the adsorption enthalpies of the oxychlorides of each element on the quartz surface from their isothermal gas chromatographic behavior. The obtained volatility sequence of the group-5 elements is found to be Nb
= 6.76 min.), respectively. We successfully determined the adsorption enthalpies of the oxychlorides of each element on the quartz surface from their isothermal gas chromatographic behavior. The obtained volatility sequence of the group-5 elements is found to be Nb  Ta
Ta 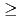 Db.
Db.
Otani, Ryo; Sato, Tetsuya; Aoki, Ryota*; Shirai, Kaori*; Suzuki, Hayato; Tsukada, Kazuaki; Asai, Masato; Ito, Yuta; Nagame, Yuichiro*; Sakama, Minoru*
no journal, ,
The adsorption enthalpy of the Sg dioxydichloride on a quartz surface (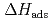 (SgO
(SgO Cl
Cl )) has been reported to be -98 kJ/mol. The result, however, is still ambiguous because the value was evaluated based on a few experimental points with large statistics errors. To obtain a reliable
)) has been reported to be -98 kJ/mol. The result, however, is still ambiguous because the value was evaluated based on a few experimental points with large statistics errors. To obtain a reliable 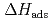 (SgO
(SgO Cl
Cl )) for a discussion of an influence of relativistic effects, a stable gas chemistry apparatus with good reproducibility is mandatory. In this study, we have conducted offline experiments of an isothermal gas chromatography) with short-lived Mo isotopes originated from a
)) for a discussion of an influence of relativistic effects, a stable gas chemistry apparatus with good reproducibility is mandatory. In this study, we have conducted offline experiments of an isothermal gas chromatography) with short-lived Mo isotopes originated from a 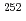 Cf fission source. We searched for experimental parameters for an on-line experiment. At the presentation, we will present the obtained optimum conditions for gas chromatographic separation of group-6 elements and determined
Cf fission source. We searched for experimental parameters for an on-line experiment. At the presentation, we will present the obtained optimum conditions for gas chromatographic separation of group-6 elements and determined 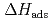 (MoO
(MoO Cl
Cl )).
)).